Yesdog
Well-Known Member
Alright guys, it's been a few months of me running around here talking about 'low P', dishing out the theories and research I've been tracking, etc. Finally wanted to post some results. I'm about 2 weeks from harvest, and 90% of this grow so far has been on a custom low P high N diet the entire grow. The only thing I changed later in flowering is adding more epsom salt (MgSO4) than I used during veg, maybe 10-20% more.
Phosphorus- everything I've read on the subject in published papers, plus some good anecdotal evidence and forum claims, seems to show that low P levels are very beneficial as far as plant health, and overall health of the chemical solution itself. Phosphates have a very interesting solution chemistry that involves ph-dependent speciation (its not always a phosphate), very similar to carbonates. Carbonates and phosphates also both tend to form low-solubility compounds with metal cations (Mg, Ca, Mo, etc). The variable speciation of these ions makes them both valuable buffers, because when ph changes the speciation starts balancing itself against certain factors such as solubility (dissociation), and additionally in the carbonate case, dissolved CO2. This is why you'll find most pH Downs are phosphate based, and pH Ups are carbonate based.
These solubility rules are also mostly what governs the "nutrient availability at a certain pH" chart. It's not that X element or Y element is less available at a certain pH, it's more that "at a certain pH, a less-dissociative compound may form". So at a certain pH where... Calcium is less available, it's actually more like: "at this pH, calcium phosphate may be less available" or "calcium sulfate may be less available". So it's a matter of 2 ions, at a strong concentration, with the right pH, then it may take on a less 'available' (dissociative) form. This also becomes more dependent on the concentration of various ions in your recipe. So a low P mix may actually have a higher Ca/Mg availability at a low pH than something with high P.
So, what does this really mean? Well, it means that every solubility/availability issues involves two parts. A low pH will make calcium less available, provided there is a strong phosphate or carbonate concentration. Well shit. So these nice buffered GH pH ups and downs are actually making that situation worse.
Remember when I mentioned the ph-dependent speciation makes them good buffers? Well, it also makes them really slow reactions. Carbonate buffers will eventually equalize with available dissolved CO2 from the air. Phosphates are also slow, and not bound by dissolved gasses, but... shit. They're also a super in-demand nutrient for the plants and microbes. As in, most bio-life will eat phosphorus as fast as it possibly can, the plants too. This means the added phosphates are a big target for any algae, bacteria, fungus, you name it. Once a microbial bloom dies, most of the phosphorus is released back into solution- giving you rather large swings of phosphorus, and opening you up to many pathogen and root issues.
So anyways, most of the papers I read stated that available soluble phosphate in soil really never gets above 30 ppm, and thats during the peak of the phosphorus cycle in soil.
Here's some P ppms for various formulas:
GrowWeedEasy DWC: 10ppm - 53ppm - 33ppm
Lucas light (5/10): 67ppm
Lucas heavy (8/16): 107ppm
GH recommend (half strength): 16ppm - 40ppm - 51ppm
Besides lucas, most of the schedules tip-toe around the 30ppm mark. I designed my formula to keep a steady 30ppm. It's similar to week 3 of the GH recommend recirculating schedule, but with less micro and more epsom. But it's basically 4ml micro, 8ml grow, 3ml bloom per gal + 0.1g epsom (all per gallon) + tap water. I kept this steady for most of the entire grow (first few weeks was something a bit different), but with more epsom salt during late flowering.
All of flowering so far as has been 1 rez change, I've only been adding water or nutrient mix (at same ratios) when ppm drops. I've been using a phosphate test kit to check for phosphate levels specifically, but I've honestly not seen em drop below 20ppm yet (highest my kit tests for). I've been treating with Great White, and dosing with Floralicious+ every few weeks (sparingly, and only with a good dose of great white).
As far as 'high N', my mix pretty much just keeps a steady 120ppm N. It's not high for veg, but it is a bit high for flowering normally.
So the main way I fixed my pH was by ditching the store-bought GH pH Up and Down. I switched to sulfuric acid and potassium hydroxide.
Potassium Hydroxide you can get like... 2 pounds of dry salts for less than $20 on Amazon. This will make about 10L of pH up at similar strength. Potassium is another super soluble ion with a high electrical-conductivity that the plants can deal with very easily. It's used internally as an 'ion-exchange' currency, it's the main electrolyte used by plant cells. Be careful when mixing this shit up, you can melt your hand off.
For the sulfuric acid, I personally just bought some reagent grade sulfuric. If you have a chemical supply house near you, you can grab some pre-made 10%. Nitric acid would be even better because nitrates have really strong solubility characteristics. Plants can deal with up to 300ppm of nitrates in solution before it's definitely toxic. Most of the N toxicity you see with premade nutrients is actually Ammonium toxicity. Ammonium can only be tolerated up to about 20ppm, so when you see nitrogen sick plants, its usually the ammonium.
Strong acids like nitric or sulfuric are also great at breaking carbonates in hard water. If you crash your ph (to like 4) with a strong acid, then bring it back up with a strong base (like potassium hydroxide) you'll have destroyed most of the carbonates in the water, and it should behave a bit more like RO (you convert the calcium carbonate into calcium nitrate/sulfate).
So, I can now officially confirm that this at least hasn't killed my plants lol. They're almost done flowering, and everything seems to be going normally. My pH doesn't creep up at all, only down. This is really the only natural way the ph should shift ever. If the plants drink more water than nutrients, the pH will drop because the solution is getting stronger. If the solution is too week, the pH should still drop. The plants natural reaction to most (except N and K i think) nutrient deficiencies is to drop the pH by exuding organic acids. This is the plants main mechanism for extracting nutrients from alkaline soil. The roots however normally rely on the ph of the soil to 'bounce' back up, so this is really the main place where manual intervention is needed with DWC/water-culture.
I've got sources for 90% of this stuff, if something looks fishy I can 90% for sure reference the source. I actually found a bunch of this info reading around about Mych research. They're very involved in the phosphorus cycle, ph management, and root exudates.
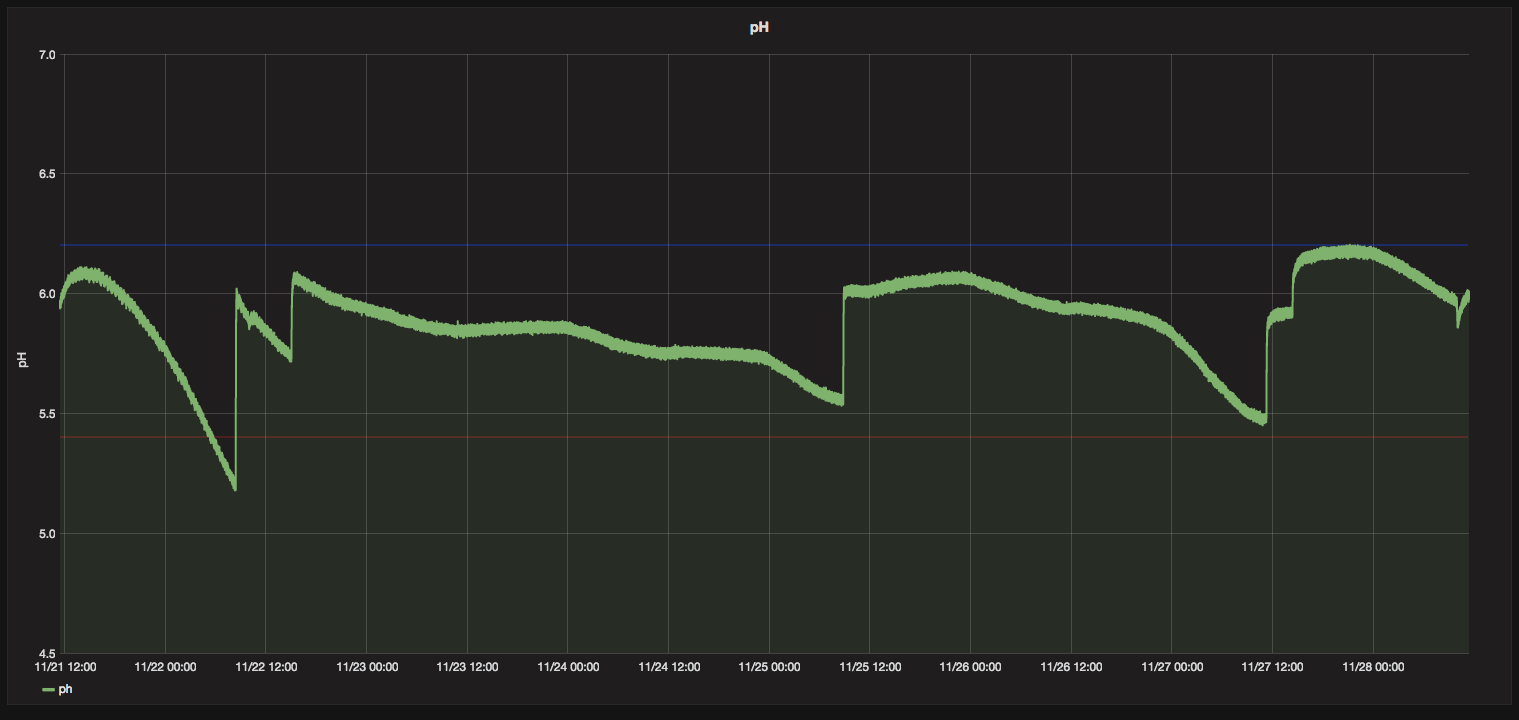
Here's what my last week of pH looked like. All the bumps up are me adding potassium hydroxide or tap water. I honestly haven't had to use any acid/down in a long while. The rez will stay stable for a few days before it drops off again. I only have 10 gal for 4 plants, so I think the exudates end up swinging the ph far more than normal.
and here's the buds so far. These are both top buds really close to the light, so they're curling a bit, but all the lower fan leaves look 100% (click to enlarge).


The second one has had reallly dark leaves since flowering. It's also easily my most aggressive plant, so I really don't think its N toxicity. The others are also a more tame color. But besides the crazy dark color, I have seen 0 nutritional oddities out of the plants since... 2 weeks after 100% switching to these feed schedule/procedure. Before i switched over I had a rough 2 weeks of flushing trying to get rid of damn phosphate precipitates caused by using too much GH pH Down (like 250ml of it in 4 weeks).
Phosphorus- everything I've read on the subject in published papers, plus some good anecdotal evidence and forum claims, seems to show that low P levels are very beneficial as far as plant health, and overall health of the chemical solution itself. Phosphates have a very interesting solution chemistry that involves ph-dependent speciation (its not always a phosphate), very similar to carbonates. Carbonates and phosphates also both tend to form low-solubility compounds with metal cations (Mg, Ca, Mo, etc). The variable speciation of these ions makes them both valuable buffers, because when ph changes the speciation starts balancing itself against certain factors such as solubility (dissociation), and additionally in the carbonate case, dissolved CO2. This is why you'll find most pH Downs are phosphate based, and pH Ups are carbonate based.
These solubility rules are also mostly what governs the "nutrient availability at a certain pH" chart. It's not that X element or Y element is less available at a certain pH, it's more that "at a certain pH, a less-dissociative compound may form". So at a certain pH where... Calcium is less available, it's actually more like: "at this pH, calcium phosphate may be less available" or "calcium sulfate may be less available". So it's a matter of 2 ions, at a strong concentration, with the right pH, then it may take on a less 'available' (dissociative) form. This also becomes more dependent on the concentration of various ions in your recipe. So a low P mix may actually have a higher Ca/Mg availability at a low pH than something with high P.
So, what does this really mean? Well, it means that every solubility/availability issues involves two parts. A low pH will make calcium less available, provided there is a strong phosphate or carbonate concentration. Well shit. So these nice buffered GH pH ups and downs are actually making that situation worse.
Remember when I mentioned the ph-dependent speciation makes them good buffers? Well, it also makes them really slow reactions. Carbonate buffers will eventually equalize with available dissolved CO2 from the air. Phosphates are also slow, and not bound by dissolved gasses, but... shit. They're also a super in-demand nutrient for the plants and microbes. As in, most bio-life will eat phosphorus as fast as it possibly can, the plants too. This means the added phosphates are a big target for any algae, bacteria, fungus, you name it. Once a microbial bloom dies, most of the phosphorus is released back into solution- giving you rather large swings of phosphorus, and opening you up to many pathogen and root issues.
So anyways, most of the papers I read stated that available soluble phosphate in soil really never gets above 30 ppm, and thats during the peak of the phosphorus cycle in soil.
Here's some P ppms for various formulas:
GrowWeedEasy DWC: 10ppm - 53ppm - 33ppm
Lucas light (5/10): 67ppm
Lucas heavy (8/16): 107ppm
GH recommend (half strength): 16ppm - 40ppm - 51ppm
Besides lucas, most of the schedules tip-toe around the 30ppm mark. I designed my formula to keep a steady 30ppm. It's similar to week 3 of the GH recommend recirculating schedule, but with less micro and more epsom. But it's basically 4ml micro, 8ml grow, 3ml bloom per gal + 0.1g epsom (all per gallon) + tap water. I kept this steady for most of the entire grow (first few weeks was something a bit different), but with more epsom salt during late flowering.
All of flowering so far as has been 1 rez change, I've only been adding water or nutrient mix (at same ratios) when ppm drops. I've been using a phosphate test kit to check for phosphate levels specifically, but I've honestly not seen em drop below 20ppm yet (highest my kit tests for). I've been treating with Great White, and dosing with Floralicious+ every few weeks (sparingly, and only with a good dose of great white).
As far as 'high N', my mix pretty much just keeps a steady 120ppm N. It's not high for veg, but it is a bit high for flowering normally.
So the main way I fixed my pH was by ditching the store-bought GH pH Up and Down. I switched to sulfuric acid and potassium hydroxide.
Potassium Hydroxide you can get like... 2 pounds of dry salts for less than $20 on Amazon. This will make about 10L of pH up at similar strength. Potassium is another super soluble ion with a high electrical-conductivity that the plants can deal with very easily. It's used internally as an 'ion-exchange' currency, it's the main electrolyte used by plant cells. Be careful when mixing this shit up, you can melt your hand off.
For the sulfuric acid, I personally just bought some reagent grade sulfuric. If you have a chemical supply house near you, you can grab some pre-made 10%. Nitric acid would be even better because nitrates have really strong solubility characteristics. Plants can deal with up to 300ppm of nitrates in solution before it's definitely toxic. Most of the N toxicity you see with premade nutrients is actually Ammonium toxicity. Ammonium can only be tolerated up to about 20ppm, so when you see nitrogen sick plants, its usually the ammonium.
Strong acids like nitric or sulfuric are also great at breaking carbonates in hard water. If you crash your ph (to like 4) with a strong acid, then bring it back up with a strong base (like potassium hydroxide) you'll have destroyed most of the carbonates in the water, and it should behave a bit more like RO (you convert the calcium carbonate into calcium nitrate/sulfate).
So, I can now officially confirm that this at least hasn't killed my plants lol. They're almost done flowering, and everything seems to be going normally. My pH doesn't creep up at all, only down. This is really the only natural way the ph should shift ever. If the plants drink more water than nutrients, the pH will drop because the solution is getting stronger. If the solution is too week, the pH should still drop. The plants natural reaction to most (except N and K i think) nutrient deficiencies is to drop the pH by exuding organic acids. This is the plants main mechanism for extracting nutrients from alkaline soil. The roots however normally rely on the ph of the soil to 'bounce' back up, so this is really the main place where manual intervention is needed with DWC/water-culture.
I've got sources for 90% of this stuff, if something looks fishy I can 90% for sure reference the source. I actually found a bunch of this info reading around about Mych research. They're very involved in the phosphorus cycle, ph management, and root exudates.
Here's what my last week of pH looked like. All the bumps up are me adding potassium hydroxide or tap water. I honestly haven't had to use any acid/down in a long while. The rez will stay stable for a few days before it drops off again. I only have 10 gal for 4 plants, so I think the exudates end up swinging the ph far more than normal.

and here's the buds so far. These are both top buds really close to the light, so they're curling a bit, but all the lower fan leaves look 100% (click to enlarge).


The second one has had reallly dark leaves since flowering. It's also easily my most aggressive plant, so I really don't think its N toxicity. The others are also a more tame color. But besides the crazy dark color, I have seen 0 nutritional oddities out of the plants since... 2 weeks after 100% switching to these feed schedule/procedure. Before i switched over I had a rough 2 weeks of flushing trying to get rid of damn phosphate precipitates caused by using too much GH pH Down (like 250ml of it in 4 weeks).
Last edited:




