PJ Diaz
Well-Known Member
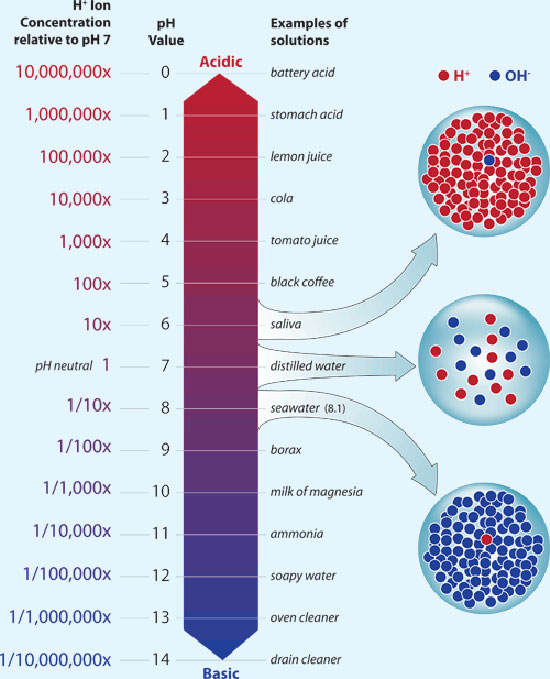
Don't worry about measuring runoff in soil grows. In hydro, it can be a usefull tool, but not for soil.So I have been watering my plants with just ph adjusted water at 6.5 ph and ppm at 160. And when I check my run off 2 plants are 6.0 ph and the other 2 are 5.1 ph and all there ppm amounts fall between 3000- 4000. Wtf that seems high But this is my first grow so Iam assuming that’s the hot soil that ppl keep talking about. Plants seem healthy besides some white dots on 2 plants and one leaf that has some burnt Can anyone help explain the ph and ppm levels Iam getting and why.
Ph adjusting plain water for soil is going to cause problems. There is no benefit as fox farm, as well as most fortified soils are buffered are the soil level. Drowning it in acid will kill all the good beneficial microbes which are feeding your plants.
I know this is not what most people say.... keepyour ph adjustments for when your feeding a nutrient solution. That’s the only time itmatters. ( with in reason) if water ph is 8plus then it’s prob a water problem to begin with.
While it's true that soil microbes will adjust soil ph, I disagree that it's not beneficial to adjust the ph to a more desirable range before feeding.This
Water with tap. Even if it’s 8. Pics
Consider this: soil microbes have to work to readjust the soil ph, while they could be doing other things. Don't you want your microbes to be working on other things for your plant, instead of adjusting the ph for you, when you could just do it yourself? Also, if the microbes are adjusting the soil to 6.5, and you add 8ph water, you are likely killing off microbes. A little ph down to get the feed to 6.5 will certainly have create a more accommodating aqueous solution for your soil life.