Which would produce more DO in a DWC?
- Thread starter JSB99
- Start date
-
- Tags
- dwc hydroponics rdwc undercurrent
Johnei
Well-Known Member
Got me thinking about how the earth transformed from co2 rich to more oxygen rich from these photosynthetic algae family type microbes. There may be a way, with extreme experimentation who knows. But my next thought is they might take over, too much. And would have to filter them out only grabbing the o2 rich water without them getting to the plants.
hmmmm....
hmmmm....
WeedFreak78
Well-Known Member
This abstract says lower O2 levels increase root mass. It would figure if thers ample O2 the roots dont need to search it out. Maybe there's benefits to running minimal O2 levels initially to promote root growth.
This line is interesting, more height without weight. They stretch more.
"Almost all the measured growth parameters (fresh and dry weights of root, stem, and leaf, leaf area, stem diameter) were significantly reduced in plants grown in the 40 mg L−1 treatment compared to plants in the lower level of DO treatments, except that the plant height increased with the increasing DO concentration."
http://www.sciencedirect.com/science/article/pii/S0304423807001203
An upper limit for elevated root zone dissolved oxygen concentration for tomato
https://doi.org/10.1016/j.scienta.2007.03.011Get rights and content
Abstract
It is well understood that insufficient oxygen within plant root zones can greatly diminish plant productivity. However, little is known about the effect of elevated root zone oxygen concentrations. Tomato (Lycopersicon lycopersicum Mill., cv. Trust) seedlings were grown in nutrient solutions containing dissolved oxygen (DO) concentration ranging from 5.3 to 40 mg L−1 for 4 weeks. There were no visible symptoms observed on the leaves or stems in any of the treatments. Leaf chlorophyll content was higher in the 40 mg L−1 treatment than with 20 and 30 mg L−1 DO treatments. Two weeks from the start of the experiment, roots in the 40 mg L−1 treatment exhibited stunted growth, became thicker, and had fewer side and fine roots compared to roots in the lower levels of DO treatment. Almost all the measured growth parameters (fresh and dry weights of root, stem, and leaf, leaf area, stem diameter) were significantly reduced in plants grown in the 40 mg L−1 treatment compared to plants in the lower level of DO treatments, except that the plant height increased with the increasing DO concentration. Root respiration increased linearly with increasing DO concentration; however, there was no effect on leaf net CO2 exchange rate. It is suggested that it was safe to enrich root zone DO to as high as 30 mg L−1, although the growth benefit was minor by increasing DO from ambient air saturated level (∼8.5 mg L−1) to 30 mg L−1. Higher than 30 mg L−1 could cause reduction in tomato plant growth
This line is interesting, more height without weight. They stretch more.
"Almost all the measured growth parameters (fresh and dry weights of root, stem, and leaf, leaf area, stem diameter) were significantly reduced in plants grown in the 40 mg L−1 treatment compared to plants in the lower level of DO treatments, except that the plant height increased with the increasing DO concentration."
http://www.sciencedirect.com/science/article/pii/S0304423807001203
An upper limit for elevated root zone dissolved oxygen concentration for tomato
https://doi.org/10.1016/j.scienta.2007.03.011Get rights and content
Abstract
It is well understood that insufficient oxygen within plant root zones can greatly diminish plant productivity. However, little is known about the effect of elevated root zone oxygen concentrations. Tomato (Lycopersicon lycopersicum Mill., cv. Trust) seedlings were grown in nutrient solutions containing dissolved oxygen (DO) concentration ranging from 5.3 to 40 mg L−1 for 4 weeks. There were no visible symptoms observed on the leaves or stems in any of the treatments. Leaf chlorophyll content was higher in the 40 mg L−1 treatment than with 20 and 30 mg L−1 DO treatments. Two weeks from the start of the experiment, roots in the 40 mg L−1 treatment exhibited stunted growth, became thicker, and had fewer side and fine roots compared to roots in the lower levels of DO treatment. Almost all the measured growth parameters (fresh and dry weights of root, stem, and leaf, leaf area, stem diameter) were significantly reduced in plants grown in the 40 mg L−1 treatment compared to plants in the lower level of DO treatments, except that the plant height increased with the increasing DO concentration. Root respiration increased linearly with increasing DO concentration; however, there was no effect on leaf net CO2 exchange rate. It is suggested that it was safe to enrich root zone DO to as high as 30 mg L−1, although the growth benefit was minor by increasing DO from ambient air saturated level (∼8.5 mg L−1) to 30 mg L−1. Higher than 30 mg L−1 could cause reduction in tomato plant growth
WeedFreak78
Well-Known Member
Just need to figure out cultivation parameters. I wouldn't think it would be much harder than keeping any other beneficial herd going. If it's adding enough o2, you might be able to run other bennies along side of it. I found a few articles about stagnant pound ecologies I'm going to look at.What will those bacteria eat?
Their food I am thinking, is same as bad stuff.
Will be hard to 'isolate' only certain types
Interesting thought, regardless.
Johnei
Well-Known Member
I have been reading lately about anaerobic bacteria that live inside the soil outdoors and provide benefits to plants, because soil for sure is not a super oxygented place for microbes all the time and plants still flourish. Rain brings oxygen as it leaches through pulling atmospheric gases behind it but there is so much anerobic life in there. I want to understand how they effect plants in beneficial way, because a lot of research is leaning to this showing that not all are destructive/pathogenic etc. Still need to read more. Basically I don' know shit about it right now. 

WeedFreak78
Well-Known Member
This is a PDF
https://www.google.com/url?sa=t&source=web&rct=j&url=http://www.indiana.edu/~clp/documents/water_column/Water_Col_V23N1.pdf&ved=0ahUKEwjX7ObUmKrVAhUF8z4KHTvGB4cQFggpMAI&usg=AFQjCNEwo1L-KW9eg6sNJIFU9iLyNo_SHQ
Oxygen –The Most Important
Water Quality Parameter?
~ Bill Jones
I’m often asked, “If you could sample only one thing on a lake, what
would it be?” For me, the answer would be dissolved oxygen. Dissolved
oxygen (DO), the measure of gaseous oxygen in water, is necessary for good
water quality. It is essential for gilled fish and insects, and influences many
different biological and chemical processes in lakes and streams.
Oxygen Properties and Dynamics
The concentration of dissolved oxygen in unpolluted fresh water
can vary greatly and is influenced by temperature, atmospheric pressure,
and salinity. For example, cold water can contain more oxygen than can
warmer water (Table 1).
Table 1. Oxygen saturation in fresh water.
Temperature (0C) Temperature (0F) Solubility (mg/L)
0 32 14.62
10 50 11.29
20 68 9.09
30 86 7.56
Oxygen (O2) enters lakes from the atmosphere through diffusion and
mixing by waves. Although diffusion from the atmosphere is a relatively
slow process, it is responsible for most of the dissolved oxygen in our lakes.
Oxygen is also produced by algae and aquatic plants as a by-product of
photosynthesis as the following process shows:
Carbon dioxide + Water + Nutrients + Light Glucose + Oxygen
Photosynthesis converts the light energy of the sun to chemical energy
that living organisms can use for life. It is said that life on Earth as we
know it would not have been possible were it not for all the excess oxygen
produced by algae in the “primordial soup” that existed on early Earth
millions of years ago.
Oxygen in lakes is consumed by:
• Respiration of fish and aquatic organisms (much like we consume
oxygen when we breathe)
• Respiration of aerobic bacteria and microbes as they decompose
dead organic materials (leaves, twigs, algae, fish, etc.) both in the
water and on the lake bottom.
• Chemical reactions, for example, the reduction of nitrate (NO3) to
ammonia (NH4) in the hypolimnion.
In respiration, the reverse process occurs where organisms use the
chemical energy formed by photosynthesis to power their bodies:
Glucose + Oxygen Carbon dioxide + Water + ENERGY
When the concentration of
DO in water is in equilibrium with
oxygen in the atmosphere, it is
called 100 percent saturated and
occurs at the concentrations shown
in Table 1. DO in biologically
productive (eutrophic) lakes can
become supersaturated when oxygen
is produced by algae or rooted
aquatic plants more quickly than it
can escape into the atmosphere. In
some cases, the DO concentration
can build up to greater than 200
percent saturation (Figure 1). When
DO concentrations exceed 110
percent saturation, harm may come
to certain fish. Excess dissolved
oxygen can lead, in rare cases, to
“gas bubble disease” in fish where
the oxygen bubbles or emboli can
block the flow of blood through
blood vessels.
On the other hand, in
biologically productive, thermally
stratified lakes with an abundance
of decaying organic material,
the oxygen consumption by
aerobic bacteria can use up much
of the available oxygen in the
hypolimnion, leading to under-
saturated conditions. If bacterial
respiration is great enough, anoxic
conditions may result. Limnologists
consider DO concentrations of less
than 1.0 mg/L to be anoxic.
https://www.google.com/url?sa=t&source=web&rct=j&url=http://www.indiana.edu/~clp/documents/water_column/Water_Col_V23N1.pdf&ved=0ahUKEwjX7ObUmKrVAhUF8z4KHTvGB4cQFggpMAI&usg=AFQjCNEwo1L-KW9eg6sNJIFU9iLyNo_SHQ
Oxygen –The Most Important
Water Quality Parameter?
~ Bill Jones
I’m often asked, “If you could sample only one thing on a lake, what
would it be?” For me, the answer would be dissolved oxygen. Dissolved
oxygen (DO), the measure of gaseous oxygen in water, is necessary for good
water quality. It is essential for gilled fish and insects, and influences many
different biological and chemical processes in lakes and streams.
Oxygen Properties and Dynamics
The concentration of dissolved oxygen in unpolluted fresh water
can vary greatly and is influenced by temperature, atmospheric pressure,
and salinity. For example, cold water can contain more oxygen than can
warmer water (Table 1).
Table 1. Oxygen saturation in fresh water.
Temperature (0C) Temperature (0F) Solubility (mg/L)
0 32 14.62
10 50 11.29
20 68 9.09
30 86 7.56
Oxygen (O2) enters lakes from the atmosphere through diffusion and
mixing by waves. Although diffusion from the atmosphere is a relatively
slow process, it is responsible for most of the dissolved oxygen in our lakes.
Oxygen is also produced by algae and aquatic plants as a by-product of
photosynthesis as the following process shows:
Carbon dioxide + Water + Nutrients + Light Glucose + Oxygen
Photosynthesis converts the light energy of the sun to chemical energy
that living organisms can use for life. It is said that life on Earth as we
know it would not have been possible were it not for all the excess oxygen
produced by algae in the “primordial soup” that existed on early Earth
millions of years ago.
Oxygen in lakes is consumed by:
• Respiration of fish and aquatic organisms (much like we consume
oxygen when we breathe)
• Respiration of aerobic bacteria and microbes as they decompose
dead organic materials (leaves, twigs, algae, fish, etc.) both in the
water and on the lake bottom.
• Chemical reactions, for example, the reduction of nitrate (NO3) to
ammonia (NH4) in the hypolimnion.
In respiration, the reverse process occurs where organisms use the
chemical energy formed by photosynthesis to power their bodies:
Glucose + Oxygen Carbon dioxide + Water + ENERGY
When the concentration of
DO in water is in equilibrium with
oxygen in the atmosphere, it is
called 100 percent saturated and
occurs at the concentrations shown
in Table 1. DO in biologically
productive (eutrophic) lakes can
become supersaturated when oxygen
is produced by algae or rooted
aquatic plants more quickly than it
can escape into the atmosphere. In
some cases, the DO concentration
can build up to greater than 200
percent saturation (Figure 1). When
DO concentrations exceed 110
percent saturation, harm may come
to certain fish. Excess dissolved
oxygen can lead, in rare cases, to
“gas bubble disease” in fish where
the oxygen bubbles or emboli can
block the flow of blood through
blood vessels.
On the other hand, in
biologically productive, thermally
stratified lakes with an abundance
of decaying organic material,
the oxygen consumption by
aerobic bacteria can use up much
of the available oxygen in the
hypolimnion, leading to under-
saturated conditions. If bacterial
respiration is great enough, anoxic
conditions may result. Limnologists
consider DO concentrations of less
than 1.0 mg/L to be anoxic.
WeedFreak78
Well-Known Member
That last link and the Koi raising link seem to show a correlation between waters nutrient content and DO levels. Is supersaturation possible with the nutrient levels we run or is it limited? I'm going to see if i can find out how synthetic fertilizers offgas.
WeedFreak78
Well-Known Member
Nanobubbles can get to that "optimal" 30mg/L mentioned earlier.
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0065339
Oxygen and Air Nanobubble Water Solution Promote the Growth of Plants, Fishes, and Mice
Abstract
Nanobubbles (<200 nm in diameter) have several unique properties such as long lifetime in liquid owing to its negatively charged surface, and its high gas solubility into the liquid owing to its high internal pressure. They are used in variety of fields including diagnostic aids and drug delivery, while there are no reports assessing their effects on the growth of lives. Nanobubbles of air or oxygen gas were generated using a nanobubble aerator (BUVITAS; Ligaric Company Limited, Osaka, Japan). Brassica campestris were cultured hydroponically for 4 weeks within air-nanobubble water or within normal water. Sweetfish (for 3 weeks) and rainbow trout (for 6 weeks) were kept either within air-nanobubble water or within normal water. Finally, 5 week-old male DBA1/J mice were bred with normal free-chaw and free-drinking either of oxygen-nanobubble water or of normal water for 12 weeks. Oxygen-nanobubble significantly increased the dissolved oxygen concentration of water as well as concentration/size of nanobubbles which were relatively stable for 70 days. Air-nanobubble water significantly promoted the height (19.1 vs. 16.7 cm; P<0.05), length of leaves (24.4 vs. 22.4 cm; P<0.01), and aerial fresh weight (27.3 vs. 20.3 g; P<0.01) of Brassica campestris compared to normal water. Total weight of sweetfish increased from 3.0 to 6.4 kg in normal water, whereas it increased from 3.0 to 10.2 kg in air-nanobubble water. In addition, total weight of rainbow trout increased from 50.0 to 129.5 kg in normal water, whereas it increased from 50.0 to 148.0 kg in air-nanobubble water. Free oral intake of oxygen-nanobubble water significantly promoted the weight (23.5 vs. 21.8 g; P<0.01) and the length (17.0 vs. 16.1 cm; P<0.001) of mice compared to that of normal water. We have demonstrated for the first time that oxygen and air-nanobubble water may be potentially effective tools for the growth of lives.
Results
Oxygen concentration was 7.7 mg/L in original normal distilled water, whereas it increased to 31.7 mg/L in oxygen-nanobubble water immediately after running nanobubble aerator with 100 L water for 30 min (Figure 2). Figure 3 shows the chronological change and distribution of number and diameter of air-bubbles in water after generation. Approximately 70% of the generated air-bubbles were smaller than 2 µm in diameter immediately after generation. Moreover, even 2.5 months after generation, approximately 60% still remained smaller than 2 µm in diameter.
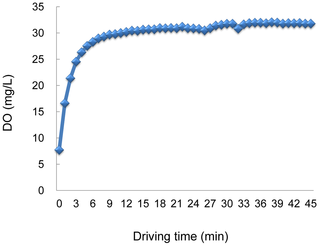
Download:
Figure 2. Sequential dissolved oxygen concentration (DO) in oxygen-nanobubble mixed water.
Figure 1 with 100 L water. Oxygen concentration was measured sequentially by Winkler's method.
https://doi.org/10.1371/journal.pone.0065339.g002

Download:
Figure 3. Sequential changes of number and diameter of generated air-nanobubbles.
Figure 1, sequential changes of number and diameter of generated air-nanobubbles were analyzed by Multisizer 3 (Beckman Coulter, Inc., Miami, FL, USA).
https://doi.org/10.1371/journal.pone.0065339.g003
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0065339
Oxygen and Air Nanobubble Water Solution Promote the Growth of Plants, Fishes, and Mice
Abstract
Nanobubbles (<200 nm in diameter) have several unique properties such as long lifetime in liquid owing to its negatively charged surface, and its high gas solubility into the liquid owing to its high internal pressure. They are used in variety of fields including diagnostic aids and drug delivery, while there are no reports assessing their effects on the growth of lives. Nanobubbles of air or oxygen gas were generated using a nanobubble aerator (BUVITAS; Ligaric Company Limited, Osaka, Japan). Brassica campestris were cultured hydroponically for 4 weeks within air-nanobubble water or within normal water. Sweetfish (for 3 weeks) and rainbow trout (for 6 weeks) were kept either within air-nanobubble water or within normal water. Finally, 5 week-old male DBA1/J mice were bred with normal free-chaw and free-drinking either of oxygen-nanobubble water or of normal water for 12 weeks. Oxygen-nanobubble significantly increased the dissolved oxygen concentration of water as well as concentration/size of nanobubbles which were relatively stable for 70 days. Air-nanobubble water significantly promoted the height (19.1 vs. 16.7 cm; P<0.05), length of leaves (24.4 vs. 22.4 cm; P<0.01), and aerial fresh weight (27.3 vs. 20.3 g; P<0.01) of Brassica campestris compared to normal water. Total weight of sweetfish increased from 3.0 to 6.4 kg in normal water, whereas it increased from 3.0 to 10.2 kg in air-nanobubble water. In addition, total weight of rainbow trout increased from 50.0 to 129.5 kg in normal water, whereas it increased from 50.0 to 148.0 kg in air-nanobubble water. Free oral intake of oxygen-nanobubble water significantly promoted the weight (23.5 vs. 21.8 g; P<0.01) and the length (17.0 vs. 16.1 cm; P<0.001) of mice compared to that of normal water. We have demonstrated for the first time that oxygen and air-nanobubble water may be potentially effective tools for the growth of lives.
Results
Oxygen concentration was 7.7 mg/L in original normal distilled water, whereas it increased to 31.7 mg/L in oxygen-nanobubble water immediately after running nanobubble aerator with 100 L water for 30 min (Figure 2). Figure 3 shows the chronological change and distribution of number and diameter of air-bubbles in water after generation. Approximately 70% of the generated air-bubbles were smaller than 2 µm in diameter immediately after generation. Moreover, even 2.5 months after generation, approximately 60% still remained smaller than 2 µm in diameter.
Download:
Figure 2. Sequential dissolved oxygen concentration (DO) in oxygen-nanobubble mixed water.
Figure 1 with 100 L water. Oxygen concentration was measured sequentially by Winkler's method.
https://doi.org/10.1371/journal.pone.0065339.g002
Download:
Figure 3. Sequential changes of number and diameter of generated air-nanobubbles.
Figure 1, sequential changes of number and diameter of generated air-nanobubbles were analyzed by Multisizer 3 (Beckman Coulter, Inc., Miami, FL, USA).
https://doi.org/10.1371/journal.pone.0065339.g003
JSB99
Well-Known Member
LMAO!I don't care about the repercussions, it's now my mission to rid this forum, and hopefully the earth, of your bullshit.
GO FUCKING KILL YOURSELF. DRINK BLEACH, STAB AN OUTLET WITH A FORK, THEN SUCK START A SHOTGUN. Is that clear enough?
The Freak is tuned up now, buzzing like an air pump wired directly into 220V A/C electrical circuit!
Great stuff for growers interested in tomatoes and DO, fish and DO is interesting, dissolved oxygen in lakes, lettuce and DO, gas supersaturation... and Nano Bubbles.
Nano bubbles are special, actually very interesting. They remain in the water column for weeks vs. floating to the surface leaving the water. Nano bubble generators vs air bubblers. Electrolysis of water also produces oxygen Nano bubbles and hydrogen Nano bubbles (1:2 respectively) so claims the O2 Grow sales Oxygenator literature.
What are Nano bubbled? http://www.nanobubbles.com/nanobubbles-2/what-are-nanobubbles/?doing_wp_cron=1501191364.3389921188354492187500#.WXpcwzaWyvR
Johnie has got to be a dirt farmer, he’s even learning about “anaerobic bacteria that live inside the soil outdoors and provide benefits to plants, because soil for sure is not a super oxygented place for microbes all the time and plants still flourish.” Atta boy Johnie, learning the science trumps luck, endless experimenting and hope in the 21st century.
Ever wondered just what the biological oxygen demand is for aerobic organisms in a DWC or how much dissolved these organisms consume and require to be healthy./ Well, thinks about it because they consume a considerable amount of dissolved oxygen continuously 24/7 as they grow and multiply.
Some of you may be or may not be interested is a little cannabis science here, especially those that lack e a firm grip on vital importance of dissolved oxygen in DWC for rhizomes and beneficial aerobic micro microbes.
“So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. Another important tool to have is an oxidation-reduction potential meter (ORP meter), which indicates the level of residual oxidizer left in the water.”
March 29, 2017 https://www.cannabisindustryjournal.com/tag/grow/
Understanding Dissolved Oxygen in Cannabis Cultivation
By Aaron G. Biros
Oxygen plays an integral role in plant photosynthesis, respiration and transpiration. Photosynthesis requires water from the roots making its way up the plant via capillary action, which is where oxygen’s job comes in. For both water and nutrient uptake, oxygen levels at the root tips and hairs is a controlling input. A plant converts sugar from photosynthesis to ATP in the respiration process, where oxygen is delivered from the root system to the leaf and plays a direct role in the process.
Charlie Hayes has a degree in biochemistry and spent the past 17 years researching and designing water treatment processes to improve plant health. Hayes is a biochemist and owner of Advanced Treatment Technologies, a water treatment solutions provider. In a presentation at the CannaGrow conference, Hayes discussed the various benefits of dissolved oxygen throughout the cultivation process. We sat down with Hayes to learn about the science behind improving cannabis plant production via dissolved oxygen.
In transpiration, water evaporates from a plant’s leaves via the stomata and creates a ‘transpirational pull,’ drawing water, oxygen and nutrients from the soil or other growing medium. That process helps cool the plant down, changes osmotic pressure in cells and enables a flow of water and nutrients up from the root system, according to Hayes.
Charlie Hayes, biochemist and owner of Advanced Treatment Technologies
Roots in an oxygen-rich environment can absorb nutrients more effectively. “The metabolic energy required for nutrient uptake come from root respiration using oxygen,” says Hayes. “Using high levels of oxygen can ensure more root mass, more fine root hairs and healthy root tips.” A majority of water in the plant is taken up by the fine root hairs and requires a lot of energy, and thus oxygen, to produce new cells.
So what happens if you don’t have enough oxygen in your root system? Hayes says that can reduce water and nutrient uptake, reduce root and overall plant growth, induce wilting (even outside of heat stress) in heat stress and reduce the overall photosynthesis and glucose transfer capabilities of the plant. Lower levels of dissolved oxygen also significantly reduce transpiration in the plant. Another effect that oxygen-deprived root systems can have is the production of ethylene, which can cause cells to collapse and make them more susceptible to disease. He says if you are having issues with unhealthy root systems, increasing the oxygen levels around the root system can improve root health. “Oxygen starved root tips can lead to a calcium shortage in the shoot,” says Hayes. “That calcium shortage is a common issue with a lack of oxygen, but in an oxygen-deprived environment, anaerobic organisms can attack the root system, which could present bigger problems.”
So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. Another important tool to have is an oxidation-reduction potential meter (ORP meter), which indicates the level of residual oxidizer left in the water.
Their treatment system includes check valves that are OSHA and fire code-compliant.
Citing research and experience from his previous work, he says that health and production improvements in cannabis plateau at the 40-45 parts-per-million (ppm) of dissolved oxygen in the root zone. But to achieve those levels, growers need to start with an even higher level of dissolved oxygen in a treatment system to deliver that 40-45 ppm to the roots. “Let’s say for example with 3 ppm of oxygen in the root tissue and 6ppm of oxygen in the surrounding soil or growing medium, higher concentrations outside of the tissue would help drive absorption for the root system membrane,” says Hayes.
Reaching that 40-45 ppm range can be difficult however and there are a couple methods of delivering dissolved oxygen. The most typical method is aeration of water using bubbling or injecting air into the water. This method has some unexpected ramifications though. Oxygen is only one of many gasses in air and those other gasses can be much more soluble in water. Paying attention to Henry’s Law is important here. Henry’s Law essentially means that the solubility of gasses is controlled by temperature, pressure and concentration. For example, Hayes says carbon dioxide is up to twenty times more soluble than oxygen. That means the longer you aerate water, the higher concentration of carbon dioxide and lower concentration of oxygen over time.
Another popular method of oxidizing water is chemically. Some growers might use hydrogen peroxide to add dissolved oxygen to a water-based solution, but that can create a certain level of phytotoxicity that could be bad for root health.
Using ozone, Hayes says, is by far the most effective method of getting dissolved oxygen in water, (because it is 12 ½ times more soluble than oxygen). But just using an ozone generator will not effectively deliver dissolved oxygen at the target levels to the root system. In order to use ozone properly, you need a treatment system that can handle a high enough concentration of ozone, mix it properly and hold it in the solution, says Hayes. “Ozone is an inherently unstable molecule, with a half-life of 15 minutes and even down to 3-5 minutes, which is when it converts to dissolved oxygen,” says Hayes. Using a patented control vessel, Hayes can use a counter-current, counter-rotational liquid vortex to mix the solution under pressure after leaving a vacuum. Their system can produce two necessary tools for growers: highly ozonized water, which can be sent through the irrigation system to effectively destroy microorganisms and resident biofilms, and water with high levels of dissolved oxygen for use in the root system.
This about Cannabis DWC DO, not Tomatoes or fish,
There’s much more info too.
Great stuff for growers interested in tomatoes and DO, fish and DO is interesting, dissolved oxygen in lakes, lettuce and DO, gas supersaturation... and Nano Bubbles.
Nano bubbles are special, actually very interesting. They remain in the water column for weeks vs. floating to the surface leaving the water. Nano bubble generators vs air bubblers. Electrolysis of water also produces oxygen Nano bubbles and hydrogen Nano bubbles (1:2 respectively) so claims the O2 Grow sales Oxygenator literature.
What are Nano bubbled? http://www.nanobubbles.com/nanobubbles-2/what-are-nanobubbles/?doing_wp_cron=1501191364.3389921188354492187500#.WXpcwzaWyvR
Johnie has got to be a dirt farmer, he’s even learning about “anaerobic bacteria that live inside the soil outdoors and provide benefits to plants, because soil for sure is not a super oxygented place for microbes all the time and plants still flourish.” Atta boy Johnie, learning the science trumps luck, endless experimenting and hope in the 21st century.
Ever wondered just what the biological oxygen demand is for aerobic organisms in a DWC or how much dissolved these organisms consume and require to be healthy./ Well, thinks about it because they consume a considerable amount of dissolved oxygen continuously 24/7 as they grow and multiply.
Some of you may be or may not be interested is a little cannabis science here, especially those that lack e a firm grip on vital importance of dissolved oxygen in DWC for rhizomes and beneficial aerobic micro microbes.
“So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. Another important tool to have is an oxidation-reduction potential meter (ORP meter), which indicates the level of residual oxidizer left in the water.”
March 29, 2017 https://www.cannabisindustryjournal.com/tag/grow/
Understanding Dissolved Oxygen in Cannabis Cultivation
By Aaron G. Biros
Oxygen plays an integral role in plant photosynthesis, respiration and transpiration. Photosynthesis requires water from the roots making its way up the plant via capillary action, which is where oxygen’s job comes in. For both water and nutrient uptake, oxygen levels at the root tips and hairs is a controlling input. A plant converts sugar from photosynthesis to ATP in the respiration process, where oxygen is delivered from the root system to the leaf and plays a direct role in the process.
Charlie Hayes has a degree in biochemistry and spent the past 17 years researching and designing water treatment processes to improve plant health. Hayes is a biochemist and owner of Advanced Treatment Technologies, a water treatment solutions provider. In a presentation at the CannaGrow conference, Hayes discussed the various benefits of dissolved oxygen throughout the cultivation process. We sat down with Hayes to learn about the science behind improving cannabis plant production via dissolved oxygen.
In transpiration, water evaporates from a plant’s leaves via the stomata and creates a ‘transpirational pull,’ drawing water, oxygen and nutrients from the soil or other growing medium. That process helps cool the plant down, changes osmotic pressure in cells and enables a flow of water and nutrients up from the root system, according to Hayes.
Charlie Hayes, biochemist and owner of Advanced Treatment Technologies
Roots in an oxygen-rich environment can absorb nutrients more effectively. “The metabolic energy required for nutrient uptake come from root respiration using oxygen,” says Hayes. “Using high levels of oxygen can ensure more root mass, more fine root hairs and healthy root tips.” A majority of water in the plant is taken up by the fine root hairs and requires a lot of energy, and thus oxygen, to produce new cells.
So what happens if you don’t have enough oxygen in your root system? Hayes says that can reduce water and nutrient uptake, reduce root and overall plant growth, induce wilting (even outside of heat stress) in heat stress and reduce the overall photosynthesis and glucose transfer capabilities of the plant. Lower levels of dissolved oxygen also significantly reduce transpiration in the plant. Another effect that oxygen-deprived root systems can have is the production of ethylene, which can cause cells to collapse and make them more susceptible to disease. He says if you are having issues with unhealthy root systems, increasing the oxygen levels around the root system can improve root health. “Oxygen starved root tips can lead to a calcium shortage in the shoot,” says Hayes. “That calcium shortage is a common issue with a lack of oxygen, but in an oxygen-deprived environment, anaerobic organisms can attack the root system, which could present bigger problems.”
So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. Another important tool to have is an oxidation-reduction potential meter (ORP meter), which indicates the level of residual oxidizer left in the water.
Their treatment system includes check valves that are OSHA and fire code-compliant.
Citing research and experience from his previous work, he says that health and production improvements in cannabis plateau at the 40-45 parts-per-million (ppm) of dissolved oxygen in the root zone. But to achieve those levels, growers need to start with an even higher level of dissolved oxygen in a treatment system to deliver that 40-45 ppm to the roots. “Let’s say for example with 3 ppm of oxygen in the root tissue and 6ppm of oxygen in the surrounding soil or growing medium, higher concentrations outside of the tissue would help drive absorption for the root system membrane,” says Hayes.
Reaching that 40-45 ppm range can be difficult however and there are a couple methods of delivering dissolved oxygen. The most typical method is aeration of water using bubbling or injecting air into the water. This method has some unexpected ramifications though. Oxygen is only one of many gasses in air and those other gasses can be much more soluble in water. Paying attention to Henry’s Law is important here. Henry’s Law essentially means that the solubility of gasses is controlled by temperature, pressure and concentration. For example, Hayes says carbon dioxide is up to twenty times more soluble than oxygen. That means the longer you aerate water, the higher concentration of carbon dioxide and lower concentration of oxygen over time.
Another popular method of oxidizing water is chemically. Some growers might use hydrogen peroxide to add dissolved oxygen to a water-based solution, but that can create a certain level of phytotoxicity that could be bad for root health.
Using ozone, Hayes says, is by far the most effective method of getting dissolved oxygen in water, (because it is 12 ½ times more soluble than oxygen). But just using an ozone generator will not effectively deliver dissolved oxygen at the target levels to the root system. In order to use ozone properly, you need a treatment system that can handle a high enough concentration of ozone, mix it properly and hold it in the solution, says Hayes. “Ozone is an inherently unstable molecule, with a half-life of 15 minutes and even down to 3-5 minutes, which is when it converts to dissolved oxygen,” says Hayes. Using a patented control vessel, Hayes can use a counter-current, counter-rotational liquid vortex to mix the solution under pressure after leaving a vacuum. Their system can produce two necessary tools for growers: highly ozonized water, which can be sent through the irrigation system to effectively destroy microorganisms and resident biofilms, and water with high levels of dissolved oxygen for use in the root system.
This about Cannabis DWC DO, not Tomatoes or fish,
There’s much more info too.
JSB99
Well-Known Member
You're hijacking my thread, dudeThe Freak is tuned up now, buzzing like an air pump wired directly into 220V A/C electrical circuit!
Great stuff for growers interested in tomatoes and DO, fish and DO is interesting, dissolved oxygen in lakes, lettuce and DO, gas supersaturation... and Nano Bubbles.
Nano bubbles are special, actually very interesting. They remain in the water column for weeks vs. floating to the surface leaving the water. Nano bubble generators vs air bubblers. Electrolysis of water also produces oxygen Nano bubbles and hydrogen Nano bubbles (1:2 respectively) so claims the O2 Grow sales Oxygenator literature.
What are Nano bubbled? http://www.nanobubbles.com/nanobubbles-2/what-are-nanobubbles/?doing_wp_cron=1501191364.3389921188354492187500#.WXpcwzaWyvR
Johnie has got to be a dirt farmer, he’s even learning about “anaerobic bacteria that live inside the soil outdoors and provide benefits to plants, because soil for sure is not a super oxygented place for microbes all the time and plants still flourish.” Atta boy Johnie, learning the science trumps luck, endless experimenting and hope in the 21st century.
Ever wondered just what the biological oxygen demand is for aerobic organisms in a DWC or how much dissolved these organisms consume and require to be healthy./ Well, thinks about it because they consume a considerable amount of dissolved oxygen continuously 24/7 as they grow and multiply.
Some of you may be or may not be interested is a little cannabis science here, especially those that lack e a firm grip on vital importance of dissolved oxygen in DWC for rhizomes and beneficial aerobic micro microbes.
“So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. Another important tool to have is an oxidation-reduction potential meter (ORP meter), which indicates the level of residual oxidizer left in the water.”
March 29, 2017 https://www.cannabisindustryjournal.com/tag/grow/
Understanding Dissolved Oxygen in Cannabis Cultivation
By Aaron G. Biros
Oxygen plays an integral role in plant photosynthesis, respiration and transpiration. Photosynthesis requires water from the roots making its way up the plant via capillary action, which is where oxygen’s job comes in. For both water and nutrient uptake, oxygen levels at the root tips and hairs is a controlling input. A plant converts sugar from photosynthesis to ATP in the respiration process, where oxygen is delivered from the root system to the leaf and plays a direct role in the process.
Charlie Hayes has a degree in biochemistry and spent the past 17 years researching and designing water treatment processes to improve plant health. Hayes is a biochemist and owner of Advanced Treatment Technologies, a water treatment solutions provider. In a presentation at the CannaGrow conference, Hayes discussed the various benefits of dissolved oxygen throughout the cultivation process. We sat down with Hayes to learn about the science behind improving cannabis plant production via dissolved oxygen.
In transpiration, water evaporates from a plant’s leaves via the stomata and creates a ‘transpirational pull,’ drawing water, oxygen and nutrients from the soil or other growing medium. That process helps cool the plant down, changes osmotic pressure in cells and enables a flow of water and nutrients up from the root system, according to Hayes.
Charlie Hayes, biochemist and owner of Advanced Treatment Technologies
Roots in an oxygen-rich environment can absorb nutrients more effectively. “The metabolic energy required for nutrient uptake come from root respiration using oxygen,” says Hayes. “Using high levels of oxygen can ensure more root mass, more fine root hairs and healthy root tips.” A majority of water in the plant is taken up by the fine root hairs and requires a lot of energy, and thus oxygen, to produce new cells.
So what happens if you don’t have enough oxygen in your root system? Hayes says that can reduce water and nutrient uptake, reduce root and overall plant growth, induce wilting (even outside of heat stress) in heat stress and reduce the overall photosynthesis and glucose transfer capabilities of the plant. Lower levels of dissolved oxygen also significantly reduce transpiration in the plant. Another effect that oxygen-deprived root systems can have is the production of ethylene, which can cause cells to collapse and make them more susceptible to disease. He says if you are having issues with unhealthy root systems, increasing the oxygen levels around the root system can improve root health. “Oxygen starved root tips can lead to a calcium shortage in the shoot,” says Hayes. “That calcium shortage is a common issue with a lack of oxygen, but in an oxygen-deprived environment, anaerobic organisms can attack the root system, which could present bigger problems.”
So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. Another important tool to have is an oxidation-reduction potential meter (ORP meter), which indicates the level of residual oxidizer left in the water.
Their treatment system includes check valves that are OSHA and fire code-compliant.
Citing research and experience from his previous work, he says that health and production improvements in cannabis plateau at the 40-45 parts-per-million (ppm) of dissolved oxygen in the root zone. But to achieve those levels, growers need to start with an even higher level of dissolved oxygen in a treatment system to deliver that 40-45 ppm to the roots. “Let’s say for example with 3 ppm of oxygen in the root tissue and 6ppm of oxygen in the surrounding soil or growing medium, higher concentrations outside of the tissue would help drive absorption for the root system membrane,” says Hayes.
Reaching that 40-45 ppm range can be difficult however and there are a couple methods of delivering dissolved oxygen. The most typical method is aeration of water using bubbling or injecting air into the water. This method has some unexpected ramifications though. Oxygen is only one of many gasses in air and those other gasses can be much more soluble in water. Paying attention to Henry’s Law is important here. Henry’s Law essentially means that the solubility of gasses is controlled by temperature, pressure and concentration. For example, Hayes says carbon dioxide is up to twenty times more soluble than oxygen. That means the longer you aerate water, the higher concentration of carbon dioxide and lower concentration of oxygen over time.
Another popular method of oxidizing water is chemically. Some growers might use hydrogen peroxide to add dissolved oxygen to a water-based solution, but that can create a certain level of phytotoxicity that could be bad for root health.
Using ozone, Hayes says, is by far the most effective method of getting dissolved oxygen in water, (because it is 12 ½ times more soluble than oxygen). But just using an ozone generator will not effectively deliver dissolved oxygen at the target levels to the root system. In order to use ozone properly, you need a treatment system that can handle a high enough concentration of ozone, mix it properly and hold it in the solution, says Hayes. “Ozone is an inherently unstable molecule, with a half-life of 15 minutes and even down to 3-5 minutes, which is when it converts to dissolved oxygen,” says Hayes. Using a patented control vessel, Hayes can use a counter-current, counter-rotational liquid vortex to mix the solution under pressure after leaving a vacuum. Their system can produce two necessary tools for growers: highly ozonized water, which can be sent through the irrigation system to effectively destroy microorganisms and resident biofilms, and water with high levels of dissolved oxygen for use in the root system.
This about Cannabis DWC DO, not Tomatoes or fish,
There’s much more info too.
Topic - Which would produce more DO in a DWC?
1st, how do you know your DO is low, unsafe? 2nd, what is your DO goal? 3rd, what DO would you like to see in your water 24/7 continuously for a few months?
... 40% DO Sat, 75% DO Sat, 90% DO sat, 100% DO sat, maybe 125% DO sat, 175% DO sat?… what DO do you want or would you like to have?
If you knew what DO actually have, it may be just fine. If the DO is low, there are ways to increase it to what ever you want the DO to be. The DO sat is always your choice.
If you are totally fixated on using ambient air as your source gas, your DO Saturation will be seriously limited by the oxygen concentration in ambient air. If you use air, it will make no difference whether you have 1 bubbler, 5 bubblers or 25 bubblers in each bucket, because air has serious limitations – see Henrys Law. The partial pressure of oxygen in ambient air at sea level is only 159 mm/kg tension + or – a mm/hg ( millimeter of mercury barometric pressure) or so..
If you really want to increase the DO, forget the air and try using a different gas with 1 bubbler a different gas like elemental oxygen (O2) or elemental Ozone (O3) and then test the DO in your water. If you want a higher DO, just increase the flow of O2 or O3 bubbling through 1 bubbler… it’s easy, ain’t nothing to it, it’s simply basic chemistry, ain’t nothing to manipulating DO Saturations if you use the right gas at 1 ATM pressure.
Try reading Understanding Dissolved Oxygen in Cannabis Cultivation tomorrow or in a few days when you’re fresh and bright.
This is very important ***** “So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. ****
1st, how do you know your DO is low, unsafe? 2nd, what is your DO goal? 3rd, what DO would you like to see in your water 24/7 continuously for a few months?
... 40% DO Sat, 75% DO Sat, 90% DO sat, 100% DO sat, maybe 125% DO sat, 175% DO sat?… what DO do you want or would you like to have?
If you knew what DO actually have, it may be just fine. If the DO is low, there are ways to increase it to what ever you want the DO to be. The DO sat is always your choice.
If you are totally fixated on using ambient air as your source gas, your DO Saturation will be seriously limited by the oxygen concentration in ambient air. If you use air, it will make no difference whether you have 1 bubbler, 5 bubblers or 25 bubblers in each bucket, because air has serious limitations – see Henrys Law. The partial pressure of oxygen in ambient air at sea level is only 159 mm/kg tension + or – a mm/hg ( millimeter of mercury barometric pressure) or so..
If you really want to increase the DO, forget the air and try using a different gas with 1 bubbler a different gas like elemental oxygen (O2) or elemental Ozone (O3) and then test the DO in your water. If you want a higher DO, just increase the flow of O2 or O3 bubbling through 1 bubbler… it’s easy, ain’t nothing to it, it’s simply basic chemistry, ain’t nothing to manipulating DO Saturations if you use the right gas at 1 ATM pressure.
Try reading Understanding Dissolved Oxygen in Cannabis Cultivation tomorrow or in a few days when you’re fresh and bright.
This is very important ***** “So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. ****
JSB99
Well-Known Member
Tapped into my bathroom's water. I love Shark Bite parts, but man, they're expensive!

My 31 gallon reservoir. I've got a float valve for it. For now it'll be hooked up to the tap, but eventually I may put some RO filters in-line. The water here is really good! Probably because we get so much rain. I looked up the info at the water station and I didn't see "chloramines" as one of the chemicals. "Chloride" was listed, but not "chloramines".
Anyone think this thing will split or flex too much filled with water? My bubble cloner has a couple pieces of wood that keep the tote from flexing. It works really well. I didn't like how the lid had a hard time fitting correctly when filled with water.


My 31 gallon reservoir. I've got a float valve for it. For now it'll be hooked up to the tap, but eventually I may put some RO filters in-line. The water here is really good! Probably because we get so much rain. I looked up the info at the water station and I didn't see "chloramines" as one of the chemicals. "Chloride" was listed, but not "chloramines".
Anyone think this thing will split or flex too much filled with water? My bubble cloner has a couple pieces of wood that keep the tote from flexing. It works really well. I didn't like how the lid had a hard time fitting correctly when filled with water.

WeedFreak78
Well-Known Member
You've lost any credibility with me long ago . Here are the lyrics to that song i posted. Pink guy STFU. I copied and pasted it in case you are deaf or something and didn't hear it. Weird how it bolded certain parts when I pasted it. 
Shut the fuck up. You're a fucking cunt, shut the fuck up. You're a fucking cunt, suck my dick. Shut the fuck up, stop being a fucking cunt, shut the fuck up, nobody even wants you here.
I just wanna let you know, you're a stupid fucking cunt. Go ahead and run your mouth pussy I don't give a fuck. You're a stupid piece of shit, you're a stupid fucking bitch. Get the fuck up off my dick.
Like please end your fucking life, please end your fucking life. I really gotta emhpasize, no one cares if you're alive. You're a fucking penis hole, grab a dick and eat it whole. I need to know if you were dropped when you were just a fetus, though? You're so fucking ugly and your face is fucking foul, jeez you're so fucking loud, can you shut your fucking mouth?
Can you?
Shut the fuck up. You're a fucking cunt, shut the fuck up. You're a fucking cunt, suck my dick. Shut the fuck up, stop being a fucking cunt, shut the fuck up, nobody even wants you here.
Close your fucking mouth. You're just really fucking dense. If you hate me why you talking you don’t make no fucking sense. Got a sad life, sad life, go to fucking hell. Are you stupid or disabled man I can’t fucking tell? You're a fucking dumb shit, you don't even run shit, get the fuck out of my face and go to hell, eat a dick.
Come and catch these hands boy, come and match these hands boy, I'm not crazy I just do it all because I can boy. I hope you fucking die in a high speed car crash. I hope you fucking fall head first and get your neck cracked. I hope you have some beautiful children that die from cancer. I hope you catch zika when your wife gets pregnant. I hope you win the lottery and die the next day, and your daughter has to see you getting lowered in your grave.
Like ahh, ohh, that was a little dark, I'm sorry, that, that was a little dark, very poor taste.
Shut The Fuck Up!
I shouldn’t have said that.
You're a fucking cunt!
Actually no I should’ve.
Shut The Fuck Up!
I shou-I didn’t say enough..
You’re a stupid cunt suck my dick. Shut the fuck up, stop being a fucking cunt. Shut the fuck up. Nobody even wants you here.
Sorry for the highjack @JSB99. I'm done.

Shut the fuck up. You're a fucking cunt, shut the fuck up. You're a fucking cunt, suck my dick. Shut the fuck up, stop being a fucking cunt, shut the fuck up, nobody even wants you here.
I just wanna let you know, you're a stupid fucking cunt. Go ahead and run your mouth pussy I don't give a fuck. You're a stupid piece of shit, you're a stupid fucking bitch. Get the fuck up off my dick.
Like please end your fucking life, please end your fucking life. I really gotta emhpasize, no one cares if you're alive. You're a fucking penis hole, grab a dick and eat it whole. I need to know if you were dropped when you were just a fetus, though? You're so fucking ugly and your face is fucking foul, jeez you're so fucking loud, can you shut your fucking mouth?
Can you?
Shut the fuck up. You're a fucking cunt, shut the fuck up. You're a fucking cunt, suck my dick. Shut the fuck up, stop being a fucking cunt, shut the fuck up, nobody even wants you here.
Close your fucking mouth. You're just really fucking dense. If you hate me why you talking you don’t make no fucking sense. Got a sad life, sad life, go to fucking hell. Are you stupid or disabled man I can’t fucking tell? You're a fucking dumb shit, you don't even run shit, get the fuck out of my face and go to hell, eat a dick.
Come and catch these hands boy, come and match these hands boy, I'm not crazy I just do it all because I can boy. I hope you fucking die in a high speed car crash. I hope you fucking fall head first and get your neck cracked. I hope you have some beautiful children that die from cancer. I hope you catch zika when your wife gets pregnant. I hope you win the lottery and die the next day, and your daughter has to see you getting lowered in your grave.
Like ahh, ohh, that was a little dark, I'm sorry, that, that was a little dark, very poor taste.
Shut The Fuck Up!
I shouldn’t have said that.
You're a fucking cunt!
Actually no I should’ve.
Shut The Fuck Up!
I shou-I didn’t say enough..
You’re a stupid cunt suck my dick. Shut the fuck up, stop being a fucking cunt. Shut the fuck up. Nobody even wants you here.
Sorry for the highjack @JSB99. I'm done.
JSB99
Well-Known Member
Tapped into my bathroom's water. I love Shark Bite parts, but man, they're expensive!

My 31 gallon reservoir. I've got a float valve for it. For now it'll be hooked up to the tap, but eventually I may put some RO filters in-line. The water here is really good! Probably because we get so much rain. I looked up the info at the water station and I didn't see "chloramines" as one of the chemicals. "Chloride" was listed, but not "chloramines".
Anyone think this thing will split or flex too much filled with water? My bubble cloner has a couple pieces of wood that keep the tote from flexing. It works really well. I didn't like how the lid had a hard time fitting correctly when filled with water.

Bump
At the peak of this emotional crisis or forum show, the Freak does fess-up and come clean admitting that he is the culprit that hijacked this thread – “Which would produce more DO in a DWC?” Now you know who hijacked your thread, you don’t have to guess and accuse.You're hijacking my thread, dude
The Freak is clearly having an emotional crisis of sorts here or maybe or just a 4th grader clown act on the playground.
It is obvious that the forum clown has an uncontrollable temper problem like a special education 9 year old with ADS + no parental social training at home in his formative years 1-5 YO. A dysfunctional social mess so to speak.
jsb99, now you knows exactly which forum joker hijacked your thread. The hijacker calls himself the "Freak" by his own omission and guilt… So Sick’um j.
The Freak demonstrated an emotional “burned out,” seriously needs sedatives and bed rest now and says he won’t be back on this thread so I guess this FREE entertainment is over.
The Freak is really funny when he flashes and jumps off the normal social behavior edge, when he loses his emotional control and acts the fool… he makes me laugh at his aberrant social behavior when he loses all control, having a fit of rage.
I know your should not laugh at fools ,but the Freak, now he is special, a joker, comedian, simple and funny.
The Freak is no doubt a @ 1 dysfunctional forum clown and all forums need a clown to laugh at.
dstroy
Well-Known Member
Topic - Which would produce more DO in a DWC?
1st, how do you know your DO is low, unsafe? 2nd, what is your DO goal? 3rd, what DO would you like to see in your water 24/7 continuously for a few months?
... 40% DO Sat, 75% DO Sat, 90% DO sat, 100% DO sat, maybe 125% DO sat, 175% DO sat?… what DO do you want or would you like to have?
If you knew what DO actually have, it may be just fine. If the DO is low, there are ways to increase it to what ever you want the DO to be. The DO sat is always your choice.
If you are totally fixated on using ambient air as your source gas, your DO Saturation will be seriously limited by the oxygen concentration in ambient air. If you use air, it will make no difference whether you have 1 bubbler, 5 bubblers or 25 bubblers in each bucket, because air has serious limitations – see Henrys Law. The partial pressure of oxygen in ambient air at sea level is only 159 mm/kg tension + or – a mm/hg ( millimeter of mercury barometric pressure) or so..
If you really want to increase the DO, forget the air and try using a different gas with 1 bubbler a different gas like elemental oxygen (O2) or elemental Ozone (O3) and then test the DO in your water. If you want a higher DO, just increase the flow of O2 or O3 bubbling through 1 bubbler… it’s easy, ain’t nothing to it, it’s simply basic chemistry, ain’t nothing to manipulating DO Saturations if you use the right gas at 1 ATM pressure.
Try reading Understanding Dissolved Oxygen in Cannabis Cultivation tomorrow or in a few days when you’re fresh and bright.
This is very important ***** “So how much dissolved oxygen do you need in the root system and how do you achieve that desired level? Hayes says the first step is getting a dissolved oxygen meter and probe to measure your baseline. The typical dissolved oxygen probe can detect from 20 up to 50 ppm and up to 500% saturation. That is a critical first step and tool in understanding dissolved oxygen in the root system. ****
No one wants to buy your shitty chinese made product which you can only run "3 hours a day". A waterfall or venturi injector costs so much less, and you can run those 24/7 which is what the op is doing anyway.
Commercial growers use venturi injectors, or waterfalls, or other solutions that use ambient air because air is free and the electricity cost of adding a venturi injector to an already existing circulating pump is negligible.
Shitty chinese made electrodes that need to be replaced every 18 months are not free, in fact you mark them up probably 10000% of materials cost.
Stop implying that you can't use ambient air to increase DO in water because that is exactly what commercial growers use. Why haven't they all switched to your miraculous product? Because it's a fucking shit idea and anyone with a mind for accounting would know to stay far away from something with an expected usable life of 18 months.
You can't increase DO past 100% while the shitty product you are selling isn't running. Remember, only "3 hours a day". After it shuts off, DO levels quickly drop back to equilibrium, and then dip below because the plants use oxygen.
I hope the next time you leave this forum and go elsewhere you stay there.
dstroy
Well-Known Member
I wouldn't fill up that tote all the way with water. If you're concerned about floor space get a cylinder shaped reservoir, like a rain barrel or trash can. Those will hold more water and shouldn't flex if you get the heavy duty kind. I got my reservoir and lid for $44, rubbermaid brute trash can.Tapped into my bathroom's water. I love Shark Bite parts, but man, they're expensive!
My 31 gallon reservoir. I've got a float valve for it. For now it'll be hooked up to the tap, but eventually I may put some RO filters in-line. The water here is really good! Probably because we get so much rain. I looked up the info at the water station and I didn't see "chloramines" as one of the chemicals. "Chloride" was listed, but not "chloramines".
Anyone think this thing will split or flex too much filled with water? My bubble cloner has a couple pieces of wood that keep the tote from flexing. It works really well. I didn't like how the lid had a hard time fitting correctly when filled with water.
JSB99
Well-Known Member
I was thinking about a barrel. I've got the space (where I didn't think I'd have it before). I'll add that to my list. Right now it's out of budget.I wouldn't fill up that tote all the way with water. If you're concerned about floor space get a cylinder shaped reservoir, like a rain barrel or trash can. Those will hold more water and shouldn't flex if you get the heavy duty kind. I got my reservoir and lid for $44, rubbermaid brute trash can.
Thx
You can't increase DO past 100% while the shitty product you are selling isn't running. Remember, only "3 hours a day". After it shuts off, DO levels quickly drop back to equilibrium, and then dip below because the plants use oxygen.
QUOTE]
You are a real life Whistle Blower!
Hate to disappoint you. I’m not a salesman, nor do I sell this oxygenator. My Grandpa, Dad or brothers don't sell it either or own the company that makes it.
You have accurately described a serious problem with this particular electrolysis type oxygenator if you are expecting this product to deliver continuous O2 24/7/for months.
This oxygenator does preform exactly as advertised on the internet and infomercials … it does generate 100% pure O2 by electrolysis of water and it makes no noise. Sounds good, an endless supply of cheap, pure 100% O2 from generated from your rez water.
You are right, Products that are not totally reliable that must be totally reliable that do not run continuously and fail to deliver a steady dependable supply of oxygen that will insure minimal safe SO saturation (100% DO Saturation in res water containing plant root balls and beneficial microbe colonies) continuously 24/7 month after month are made, marketed and sold to gullible buyers every day, that’s normal.
You are exactly right, When this machine is not running, when it is off, it does not produce any pure oxygen. When it is on and running it does produces very little volume supplemental oxygen and twice as much hydrogen. The machine actually runs very little in cold water as you have pointed out because water temperature is what cycles the machine on and off. Using it in cold water shortens the on cycle time, a design problem and you have identified this technical problem. If the water temperature was 9o F or greater, the machine bight stat on and produce a little more oxygen maybe 6 hours in a 24 hour period… still not enough oxygen continuously. Therein lies a major technical problem with this particular piece of life support equipment.
You are right again… When there is no additional supplemental oxygen produced by this machine when the machine is “off”, the DO does drop down to ambient O2 equilibrium very quickly.
Yes, again you are right… The low oxygen problems begin when the DO saturation in the water falls dramatically below saturation because more plants roots and more aerobic microbes in the water use or consume O2 continuously 24/7’ Like people, they need their O2 too and will surely suffocate and die without a continuous minimal safe supply of it. Aerobic metabolism consumes a substantial amount of oxygen.
Very few growers are aware of this before they buy this, only later after they buy this becomes crystal clear. You know... secret stuff.
