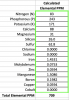
I don't believe the NPK % is the same as resulting elemental PPM ratios.
Right, well N is straightforward but others aren't.
Fertilizers are usually labeled with three numbers, as in 18-20-10, indicating the relative content of the
macronutrients nitrogen (N), phosphorus (P), and potassium (K), respectively.
More precisely, the first number ("N value") is the percentage of elemental nitrogen by weight in the fertilizer; that is, the
mass fraction of nitrogen times 100. The second number ("P value") is the percentage by weight of
phosphorus pentoxide P2O5 in a fertilizer with the same amount of phosphorus that gets all of its phosphorus from P2O5. The third number ("K value") is analogous, based on the equivalent content of
potassium oxide K2O.
[3]
For example, a 15-13-20 fertilizer would contain 15% by weight of nitrogen, and the same amounts of phosphorus and potassium as a mixture of 13% by weight of P2O5, 20% K2O, and 67% of some inert ingredient.
The values in an NPK fertilizer label are related to the concentrations (by weight) of phosphorus and potassium elements as follows:
- P2O5 consists of 56.4% elemental oxygen and 43.6% elemental phosphorus by weight. Therefore, the elemental phosphorus percentage of a fertilizer is 0.436 times its P value.
- K2O consists of 17% oxygen and 83% elemental potassium by weight. Therefore, the elemental potassium percentage is 0.83 times the K value.
The N value in NPK labels represents actual percentage of nitrogen element by weight, so it does not need to be converted.
So, for example, an 18−51−20 fertilizer contains by weight
- 18% elemental nitrogen,
- 0.436 × 51 = 22% elemental phosphorus, and
- 0.83 × 20 = 17% elemental potassium.
As another example, the fertilizer
sylvite is a naturally occurring mineral consisting mostly of
potassium chloride, KCl. Pure potassium chloride contains one potassium atom (whose
atomic mass is 39.09
g/
mol) for every chlorine atom (whose atomic mass is 35.45 g/mol). Therefore, pure KCl is 39.09/(39.09 + 35.45) = 52% potassium and 48% chlorine by weight. Its K value is therefore 52/0.83 = 63; that is, a fertilizer that gets all its potassium from K2O and has the same potassium contents as pure KCl would have to be 63% K2O. Pure KCl fertilizer would thus be labeled 0-0-63. Since sylvite contains other compounds that contribute no N, P, or K, it is usually labeled 0-0-60.