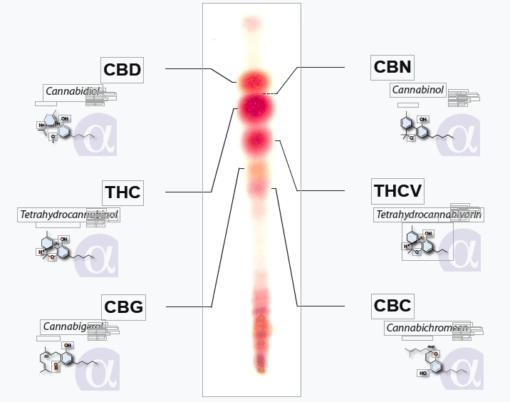
just a few

will do my best to answer them
I'm not sure if they'd work... I would assume they would, but the description has this part which I find confusing: "Activity: 3 color separated (BI-methy1 yellow,sultan red and indigo) "
To me it suggests they may be pre-treated, but i'm not sure.
I'd keep searching.
There are two main ways to view the results of TLC plates -- UV light, or provide your own 'light' in the form of a stain (like Fast Blue BB). I'm not sure if that 3-color-activity thing is in relation to UV or not sorry, but it's not the sort of thing you find in descriptions of regular plates. When you buy plates they should state if theyre UV or regular.
I just contacted a local lab supplies company and asked "do you have a small bottle of chloroform suitable for Thin Layer Chromatography?"
No, it's because I didn't get chloroform until later on. I actually prefer just using chloroform, because it's easier than measuring and dealing with 2 liquids, plus you can then avoid the horrid nasal assault of diethyl ether. Having said that though, it is awesome to have TWO different separation methods (chloroform, and diethyl+hexane) because they both help make each others results clearer to interpret.
Yes i think i paid about $40 for half a litre of chloroform.
You'll go through quite a bit of hexane because you also use that for the extraction phase (which is the most expensive phase for eluents), but yes hardly any diethyl ether is used. I only say "250mL - 1L" because those are generally the smallest sizes available.
it's called "Hexane Fraction (AR)". i'm not sure if n-hexane is different to hexane sorry. If youre not sure just ask your lab supply company for "hexane suitable for Thin Layer Chromatography". I don't know which other eluents are suitable for the extraction phase, but hexane works awesomely.
correct. The original recipe for Beam's test asks for pure ethanol, but it turns out that the ~2-5% of adulterants added to methylated spirits (simply to prevent us drinking it) doesn't seem to interfere with or affect the result of Beam's test. If this is indeed holds true (as it seems to be) it means Beam's test is an incredibly inexpensive and accessible test anyone can do at home.
be smart about the keywords you search for. It's out there. Easiest way might actually be to simply ask your local lab suppliers to see if they can source it for you - worked for me. I think i paid about $250ish for 5gm, which is actually quite a lot because you only use a few pinheads worth per test (which is good for a few plates). Just keep in mind you should be receiving it packed in dry ice, and will need to keep it in your freezer.
Best of luck, and please share anything you learn

WE NEED MORE PEOPLE MAKING THIS ACCESSIBLE!