DIY-HP-LED
Well-Known Member
Today marks the introduction of treatment options in the fight against covid -19 with the preliminary announcement by Dr. Fauci about remdesivir. Other treatment options available soon may also include convalescent plasma and there will be announcements on this soon too. There are a couple of other repurposed drugs that also show promise and these are being evaluated as well. The tool kit of treatment options available to doctors will continue to grow, as will their use. Hopefully by the end of summer these treatments used alone or in combination can help even the most severe cases to recover. If we can drive down the mortality rate and hospital resource requirements, we can open up the economy more. If these treatments are used with testing, limited contact tracing and appropriate NPI's, they offer a way forward, even to ethical herd immunity, if we can keep mortality rates low enough while doing it.
------------------------------------------------------------------------------------------------------------------------------------------------------------------
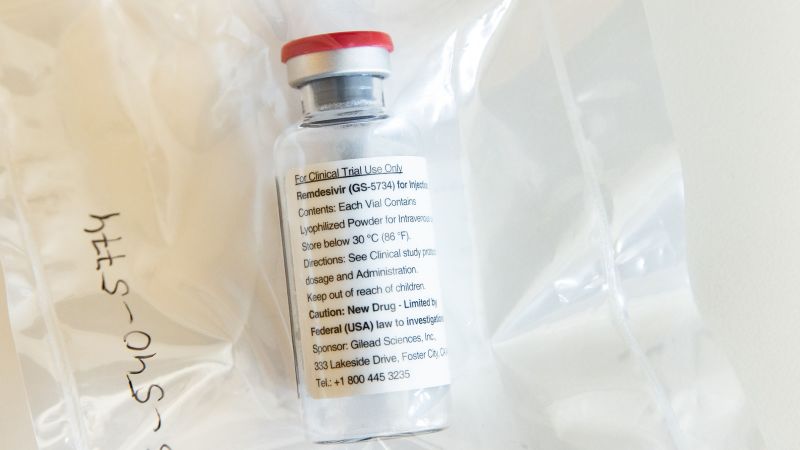
NYT: FDA plans to issue emergency authorization for remdesivir
 www.cnn.com
www.cnn.com
Fauci expresses optimism about 'significant, positive' data from a trial of the possible Covid-19 treatment remdesivir
(CNN)New data suggests patients with severe Covid-19 who took remdesivir could recover faster than patients who didn't take it, the National Institute of Allergy and Infectious Diseases said Wednesday.
"The data shows that remdesivir has a clear-cut, significant, positive effect in diminishing the time to recovery," said the institute's director, Dr. Anthony Fauci.
Results from the preliminary trial show remdesivir improved recovery time for coronavirus patients from 15 to 11 days.
"Although a 31% improvement doesn't seem like a knockout 100%, it is very important proof of concept," Fauci said. "What it has proven is that a drug can block this virus."
"Results also suggested a survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir versus 11.6% for the placebo group," the NIAID said.
Normally, data about a drug's efficacy wouldn't be released this early from a preliminary trial.
But "whenever you have clear-cut evidence that a drug works, you have an ethical obligation to immediately let the people in the placebo group know so that they can have access," Fauci said.
The US Food and Drug Administration has not yet approved any drugs for the treatment of the coronavirus.
But the FDA plans to announce an emergency-use authorization for remdesivir, according to The New York Times. The authorization could come as soon as Wednesday, the Times reported, citing a senior administration official.
n a statement to CNN, the FDA said it is in talks with Gilead Sciences, the maker of remdesivir, about making the drug available to patients.
"As part of the FDA's commitment to expediting the development and availability of potential COVID-19 treatments, the agency has been engaged in ... discussions with Gilead Sciences regarding making remdesivir available to patients as quickly as possible, as appropriate," FDA spokesman Michael Felberbaum said in statement.
Track coronavirus cases and deaths in each state and across the US
The remdesivir trial marks the first clinical trial launched in the US to evaluate an experimental treatment for Covid-19.
About 1,090 people participated in the trial internationally, Fauci said, calling it "the first truly high-powered randomized placebo controlled trial."
more...
------------------------------------------------------------------------------------------------------------------------------------------------------------------
NYT: FDA plans to issue emergency authorization for remdesivir

FDA will reportedly authorize use of remdesivir for Covid-19 after trial shows 'positive effect' on recovery time | CNN
Researchers released some good news about a possible treatment for coronavirus Wednesday -- evidence that the experimental drug remdesivir might help patients recover more quickly from the infection.
Fauci expresses optimism about 'significant, positive' data from a trial of the possible Covid-19 treatment remdesivir
(CNN)New data suggests patients with severe Covid-19 who took remdesivir could recover faster than patients who didn't take it, the National Institute of Allergy and Infectious Diseases said Wednesday.
"The data shows that remdesivir has a clear-cut, significant, positive effect in diminishing the time to recovery," said the institute's director, Dr. Anthony Fauci.
Results from the preliminary trial show remdesivir improved recovery time for coronavirus patients from 15 to 11 days.
"Although a 31% improvement doesn't seem like a knockout 100%, it is very important proof of concept," Fauci said. "What it has proven is that a drug can block this virus."
"Results also suggested a survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir versus 11.6% for the placebo group," the NIAID said.
Normally, data about a drug's efficacy wouldn't be released this early from a preliminary trial.
But "whenever you have clear-cut evidence that a drug works, you have an ethical obligation to immediately let the people in the placebo group know so that they can have access," Fauci said.
The US Food and Drug Administration has not yet approved any drugs for the treatment of the coronavirus.
But the FDA plans to announce an emergency-use authorization for remdesivir, according to The New York Times. The authorization could come as soon as Wednesday, the Times reported, citing a senior administration official.
n a statement to CNN, the FDA said it is in talks with Gilead Sciences, the maker of remdesivir, about making the drug available to patients.
"As part of the FDA's commitment to expediting the development and availability of potential COVID-19 treatments, the agency has been engaged in ... discussions with Gilead Sciences regarding making remdesivir available to patients as quickly as possible, as appropriate," FDA spokesman Michael Felberbaum said in statement.
Track coronavirus cases and deaths in each state and across the US
The remdesivir trial marks the first clinical trial launched in the US to evaluate an experimental treatment for Covid-19.
About 1,090 people participated in the trial internationally, Fauci said, calling it "the first truly high-powered randomized placebo controlled trial."
more...
Last edited: