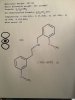
There's actually a separate forum for "concentrates and extracts", it's just way down the list. I tried using hydroxide on fresh buds before and nothing reacted even with heat, but I didn't grind them up first. How did you grind fresh bud, with a blender? It works if you make extract, like with alcohol, and then dissolve the extract with minimal alcohol, just enough to dissolve it, then slowly mix in the hydroxide solution, just a little at a time. You're left with a solution which is mostly water because the small amount of alcohol is diluted so much at that point that it's not even noticeable. Then when you add citric acid it precipitates out. If you use potassium carbonate instead of hydroxide then when you add the citric acid it forms a sponge of THCA, expands right up from the CO2 being formed. Use a large container because it really expands a lot. It shrinks back down as the water drains out though, in a filter, but it's still sponge just not blown right up like at first. After rinsing and drying it, you have a nice solid sponge THCA product, like sponge toffee but THCA. It's crispy. It gradually gets brownish from exposure to air but starts out white. It's in chunks not one big sponge.