blu3bird
Well-Known Member
People will be drinking that shit! Looks like the main ingredient of a Trump cocktail, they use iso for a reason, not because its cheaper either!
I hope no one drinks that hand sanitizer lol
People will be drinking that shit! Looks like the main ingredient of a Trump cocktail, they use iso for a reason, not because its cheaper either!
LOL A gallon of gasoline is only $2.






continued from above:
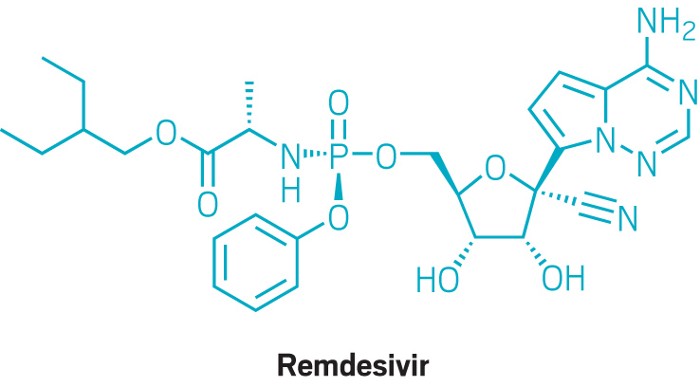
And when it comes to remdesivir, experts say Gilead might not have been inclined, or even had the time, to make the process as efficient as it could be. Gilead developed the compound during the 2014 Ebola virus outbreak in West Africa and sped it along with the goal of testing it before the outbreak waned. The process likely reflects the anticipated demand for an Ebola treatment: the 2014 Ebola outbreak saw 29,000 cases over 2.5 years; the current coronavirus pandemic is approaching 2.5 million cases in under 5 months.
As the scope of the pandemic widens, Gilead has been open about the challenges of manufacturing the drug. The firm says it typically takes 9 to 12 months to make an antiviral like remdesivir, but that since January it has shrunk the timeline to 6 to 8 months. “We continue to work on optimizing the chemical synthesis processes,” the company states.
In a public communication in early April, CEO Daniel O’Day outlined a series of measures Gilead was taking to increase access to remdesivir. At the time, the company said it had on hand enough active ingredient to create about 1.5 million doses, enough for roughly 140,000 treatment courses, based on a 10-day regimen. The company has been working to increase both internal capacity and external partnerships to generate an additional 500,000 courses by October, 1 million courses by the end of the year, and, if needed, several million courses in 2021.
The ramp up is significant. But global demand, which could include both treatment for the ongoing pandemic and government stockpiling, could dwarf those figures if the drug proves to be beneficial in treating COVID-19.
Manufacturing experts point to the astronomical demand for government stockpiles of the influenza treatment Tamiflu amid the H1N1 outbreak, which began in 2004. The situations have important differences—Tamiflu is oral, while remdesivir is intravenous, meaning its utility, if proven, would be more limited—but the thought exercise for how to tackle the problem is probably similar.
Indeed, David LaPre, who led the team at Roche that scaled up Tamiflu production, says the first question for companies with promising COVID-19 treatments is how many doses they will need. “There were a lot of debates as to what’s the right number, and I’m sure that’s the problem people are facing today as they think about antivirals as a weapon in our arsenal against COVID-19,” says LePre, who is now a pharmaceutical consultant. “I sense that now, like then, there’s not going to be a precise number—you basically have to pick one that makes sense between your own thinking and the thinking of health authorities. That becomes the target.”
In 2005, Roche based its Tamiflu target on several case studies that pegged demand at more than 1 billion doses, LaPre says.
Another layer of complexity is the vast network of partners—the bucket brigade—involved in taking a drug from raw materials to a finished product delivered to a hospital or pharmacy. Decades ago, big pharma firms performed many of those steps in-house across a handful of manufacturing sites. During the late 1990s, when Merck was preparing for a surge in demand for the HIV treatment Crixivan, “we picked up every available piece of equipment we needed” from sites in several states, Reider, who worked on Crixivan, recalls.
Roche, which licensed Tamiflu from Gilead in 1996, also was able to expand its internal capacity while at the same time building an external supply chain. Between 2005 and 2007, Roche ended up providing roughly 200 million courses of Tamiflu for government stockpiles around the globe.
But over the past 15 years, drug companies have scaled back their internal capacity. “People don’t recognize how many pieces are touched” by a vast network of contract manufacturing partners around the globe, says James Bruno, president of the consulting firm Chemical and Pharmaceutical Solutions. And “if one of those pieces fails,” it creates a bottleneck that is felt down the entire bucket brigade.
Gilead typically outsources the synthesis of its drugs through late-stage intermediates, performing the last critical steps in-house. And while it says it is trying to increase its own capacity, its first step was likely “to quickly scan the globe as to who has the right capability that can be brought to bear,” LaPre says.
I figure as soon as it's proven to work that Donald will seize the supply and use the power of life and death to control the states, he will probably ban exports and hord the drug, even if its not required, it will be power and Donald will want it. Poor countries will be shown no consideration and given nothing, unless they have dirt on Biden. The smart move would be, that if efficacy was proven for remdesivir, to have a manhattan project and produce billions of doses free for the world, it's the world economy that has to improve for the American economy to do well.We have to temper all of this with the knowledge that FEMA will likely seize all shipments of this drug in this country and do what it has been doing with ppe and ventilators. Mainly, rewarding trump allies and punishing his critics. Many states won't see more than a dribble of the drug at enormous cost while others will get all they want.
No treatment or vaccine that is in limited supplies will help the vast majority of this country recover. Period.
Or he will just say he is going to do that because he is uber aware that he can say anything to the press and it doesn't matter in a court of law.I figure as soon as it's proven to work that Donald will seize the supply and use the power of life and death to control the states, he will probably ban exports and hord the drug, even if its not required, it will be power and Donald will want it. Poor countries will be shown no consideration and given nothing, unless they have dirt on Biden. The smart move would be, that if efficacy was proven for remdesivir, to have a manhattan project and produce billions of doses free for the world, it's the world economy that has to improve for the American economy to do well.
From what I've seen thus far, it is my opinion, that using both remdesivir and convalescent plasma transfusions, even the most vulnerable can be successfully treated. If we do NPI's properly, test, isolate and contact trace, we could have an ethical plan for herd immunity. This also will greatly diminish the requirements for ICU beds and ventilators. Most people will tolerate being sick, but not being fucked for life or dead! Remove the fear and the economy will open up, but without mass gatherings for a while.
As long as Mitch and the GOP can get it themselves, fuck everybody else, I believe access to the therapies mentioned above and confidence in them by the elites is driving the opening up agenda forward, their fear is being diminished, but not yours. This drug alone has the same potential in fighting this viral infection, that penicillin had in fighting bacterial ones. From the animal studies and limited human trial information I've seen, it appears to be extremely effective. Production and the abuse of distribution by Trump appear to be the only issues that I can see. We will need to wait for further data and studies, but I'm pretty optimistic as are most experts.Or he will just say he is going to do that because he is uber aware that he can say anything to the press and it doesn't matter in a court of law.
It's why he has lost virtually everyone with any credibility from his administration/executive branch, when they drew a line, or got caught, he dumped them. He only gets press because he is the POTUS.
My fear is not being diminished? I try to be respectful mostly, but this really sounds like you are just talking shit about me.I believe access to the therapies mentioned above and confidence in them by the elites is driving the opening up agenda forward, their fear is being diminished, but not yours.
Eh? This is a potential way out of this mess, not a political talking point and not an insult to you! If these treatments can lower the infection rate to flu levels it will make a difference, a big fucking difference. I'm not here on this topic with a political agenda, but with a humanitarian one. You seem to conflate everything into some hidden agenda and if Donald breaths a word of it it must be false.My fear is not being diminished? I try to be respectful mostly, but this really sounds like you are just talking shit about me.
I don't claim to know what you are or why you post the things you do.Eh? This is a potential way out of this mess, not a political talking point and not an insult to you! If these treatments can lower the infection rate to flu levels it will make a difference, a big fucking difference. I'm not here on this topic with a political agenda, but with a humanitarian one. You seem to conflate everything into some hidden agenda and if Donald breaths a word of it it must be false.
Read the studies that I've posted, if you are not at least cautiously optimistic you don't understand the implications of either threarapy. I'm looking for ways out of this mess and sitting on our asses at home is only part of the solution. Sometime people post stuff because they actually believe it, unless you think I'm a Russian with a nefarious purpose. This is about finding the truth not about winning arguments, I stick to science and just layer on some hope and optimism. Don't bother with giving me bullshit about selling false hope either, all the facts are on this thread for any adult to make up their own mind about it. It requires a clear and unbiased mind though.
I'm selling hope, Trump and that bunch are selling bullshit, destruction and despair. I'm not talking shit about you or anybody else, its not my game, unless they are Trumpers and I have no clue how you came to such a conclusion. Reading people here try to talk about science is amusing sometimes because many are emotional basket cases who sprinkle almost every conversation with paranoia, ad hominem attacks and personal insults.
I can't fix your fear only you can do that.
