Illumination
New Member
I say growth tips....
Namaste'
Namaste'


what he said^^









Wait a minute you guys - I asked you what you wanted me to explain and you both said "yes please". Now I am confused.
Ok,
My best friend is my mag stirrer. I can use it to make media easier than anything. You just set the thing up, add the liquid, add the sugar and the MS and adjust the Ph to 5.80. Don't use paper strips and be exact, I have found that a tenth to either direction inhibits growth. The thing about the stirrer is that it is easy to adjust the Ph because a drop is dispersed in seconds.
My other best friend is my ultrasonic cleaner. A drop of detergent in with 5 percent bleach in the cleaner is about as thorough as you can get. The vibrations get inside the tiny hairs and it strips off of those persistent little bubbles that tend to stick to the cutting even when it is shaken or stirred.
Get a Big pressure cooker, they aren't that expensive and you can put enough jars of water to sterilize, plus your instruments, plus your napkins and work plate. If you are doing media, the bigger one will do 40 at a time easily (getting them out of the cooker efficiently something I haven't yet mastered).
A good bottle brush is very nice.
A good garbage disposal is tremendously helpful. You put a lot of this gel down the sink, unless it is spun up fine it will clog your pipes and you really don't want that. Many bag the stuff up and throw it out rather than risk such clogs. I would rather keep the disposal running as I dump jar after jar into it's spinning maw.
nitrile gloves
A wonderful little device is an ultrasonic denture cleaner. It is a battery operated sub-ultrasonic cleaner. It sets up standing waves in the liquid that is great for initial washes of dirty plantlets. I have also used it for bleach and alcohol washes to great effect. If I were to want to do this on a shoestring I would keep this over the full ultrasonic - AND the mag stirrer. They are something like 10 dollars each. So I would consider getting one for each phase of sterilization (well that really isn't the best word for it).
crawling insect killer - spray it on your counters once every two or three weeks
Some lab glass - a liter and a two liter and a half a liter Erlenmeyer. A couple of graduated cylinders.
Premoistened antiseptic wipes - handy for spills, quick wipedowns and such. Get a container for a buck. Speaking of which, make the 99 cent store your second home for a bit if you want to do this cheap. I laugh and laugh when I see admonitions from the experts -
"oh no, you need thousands and thousands of dollars for a lab and ultra sterile conditions, you can't do this, it is too expensive" I've had people tell me that I would need 10 thousand dollars just to start.
Of course the prime big boy toy is a decent laminar air flow hood, except when you open a contaminated jar in front of it. I have shown though and will continue to show that this is not necessary. All that you need is a good biocide and good technique and a willingness to accept a surprisingly small increase in losses. This, if you decide to get it is about $500 for a little one - and $1500 for a single person nice one that doesn't cramp your style.
A series of RACKS - racks that hold your jars or tubes or tubs. Very handy but not necessary
If you are doing this from scratch you will need another set of items - graduated pipettes, a good milligram scale, a measuring boat, a fine paintbrush (when you are working in thousandths of a gram, you can easily leave a significant portion of your powder in the boat unless you brush it out.
I didn't put this in the essentials I don't think, I'll check but you MUST have distilled water. Again, when you are working with something that works in the 1 to 10 ppm range you don't want RO water that has residuals of - 10 to 15 ppm. You want something with nothing in it but... water. I am going to finish my transfers in a few minutes. I believe, since I am in the mood to write, that I will describe the exact method of cutting and sterilizing plantlets - with pictures. Won't that be fun?




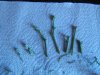
 It is important that you arrange your setup. Fluidity and efficiency is key to this procedure. Notice that the explants are in the jar on the left, my instruments are on the right in a jar full of denatured alcohol or, in this case 91 percent isopropal. I have two tweezers and two scalpels, I alternate. After each procedure I place the instrument back in the jar and take the other. This allows more time in contact with the antiseptic. I do not use flame. Any excess alcohol on the blade or tweezers is blotted off briefly on the sterile towel which is on my ceramic, sterile workplate (99 cent store sushi plate - great).
It is important that you arrange your setup. Fluidity and efficiency is key to this procedure. Notice that the explants are in the jar on the left, my instruments are on the right in a jar full of denatured alcohol or, in this case 91 percent isopropal. I have two tweezers and two scalpels, I alternate. After each procedure I place the instrument back in the jar and take the other. This allows more time in contact with the antiseptic. I do not use flame. Any excess alcohol on the blade or tweezers is blotted off briefly on the sterile towel which is on my ceramic, sterile workplate (99 cent store sushi plate - great). I have found that the majority of infection is in the fibers at the end of the cut places. If we use a sterile scalpel and slice off just a bit of the already bleach damaged end we improve our results.
I have found that the majority of infection is in the fibers at the end of the cut places. If we use a sterile scalpel and slice off just a bit of the already bleach damaged end we improve our results.






 I have always found this to be difficult, you must try not to have to adjust the angle of the plantlet after you have already picked it up. If you must touch the plantlet to something in order to adjust it's position, use the inside rim of the jar itself.
I have always found this to be difficult, you must try not to have to adjust the angle of the plantlet after you have already picked it up. If you must touch the plantlet to something in order to adjust it's position, use the inside rim of the jar itself.











D
For getting them out of the pressure cooker maybe you could find a similar sized pasta cooker (straining pot fits inside stock pot) and use the strainer (the bottoms are flat) to hold your jars, then you just lift the whole thing out when done.
One the water: my RO water comes out around 5ppm initially, then works its way up to 9 or so when the membrane is close to the end of its life. Do you think that would work?
No sir. I am sorry but even at 5 you are competing with the hormones. I don't know what is in your water and it may well be just fine but why take the chance? Pay the buck and a quarter a gallon.
1. Wrap your instruments - a couple of scalpels, a few sets of tweezers, a pair of scissors in some aluminum foil.
2. Fill at least 5 decent sized jars with tap water (although I use RO because my tap water is so absolutely crappy), cover then (foil if you must)
3. Cut some slices of paper napkin, or paper towel into squares, lay them flat and wrap that in aluminum foil as well.
4. Put all these things in your pressure cooker, bring to temp and pressure (15 lbs) and cook for 20 minutes. Allow time for everything to cool.
....
A primer on Growth hormones then?
Great thread, I just found it...I could be of some help if you need any. I have done this many times...but never with weed itself. Of course it is easier in a sterile lab environment. One thing I noticed si that your cuttings seem to be to big; you only need thin slices of the apical meristems which are where the nodes and alternate branches form. You could take severall cuttings from the ones you have use.
Also, you need horomone additives like IAA, some form of auxin/cytokine. Also, if you use cubes instead of test tube the plants can actually grow out big enough to be transplanted. This is mainly suited for leav tissue culturing. you can literally use one finger of a leaf and wash with alcohol and bleach solutions as u've done and disect it into tiny square pieces as you would in skin tissue culturing. Photos are better then words...
