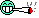Water is water right? Wrong. I see a lot of growers that have all this knowledge about nutrients and photoperiods but know very little about the water they use and that not all water is created equal and some water isn't very well suited for growing at all. I decided to do this thread to help educate growers about the water they use and why it's important to understand "The most essential compound".
What is "Hard Water"?
Perhaps you have on occassion noticed mineral deposits on your cooking dishes, or rings of insoluble soap scum in your bathtub. These are not signs of poor housekeeping, but are rather signs of
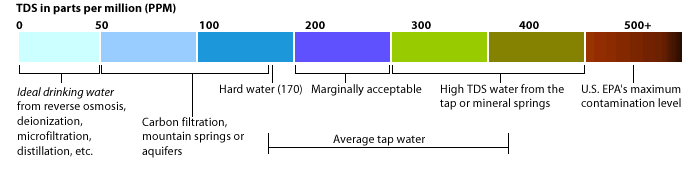
hard water from the municipal water supply. Hard water is water that contains cations with a charge of +2, especially Ca2+ and Mg2+. These ions do not pose any health threat, but they can engage in reactions that leave insoluble mineral deposits. These deposits can make hard water unsuitable for many uses, and so a variety of means have been developed to "soften" hard water;
i.e.,remove the calcium and magnesium ions.
Problems with Hard Water
Mineral deposits are formed by ionic reactions resulting in the formation of an insoluble precipitate. For example, when hard water is heated, Ca2+ ions react with bicarbonate (HCO3-) ions to form insoluble calcium carbonate (CaCO3), as shown in Equation 1.
(1)
This precipitate, known as
scale, coats the vessels in which the water is heated, producing the mineral deposits on your cooking dishes. In small quantities, these deposits are not harmful, but they may be frustrating to try to clean. As these deposits build up, however, they reduce the efficiency of heat transfer, so food may not cook as evenly or quickly in pans with large scale deposits. More serious is the situation in which industrial-sized water boilers become coated with scale: the cost in heat-transfer efficiency can have a dramatic effect on your power bill! Furthermore, scale can accumulate on the inside of appliances, such as dishwashers, and pipes. As scale builds up, water flow is impeded, and hence appliance parts and pipes must be replaced more often than if Ca2+ and Mg2+ ions were not present in the water.
Some Strategies to "Soften" Hard Water
For large-scale municipal operations, a process known as the "lime-soda process" is used to remove Ca2+ and Mg2+ from the water supply. Ion-exchange reactions, similar to those you performed in this experiment, which result in the formation of an insoluble precipitate, are the basis of this process. The water is treated with a combination of slaked lime, Ca(OH)2, and soda ash, Na2CO3. Calcium precipitates as CaCO3, and magnesium precipitates as Mg(OH)2. These solids can be collected, thus removing the scale-forming cations from the water supply.
To see this process in more detail, let us consider the reaction for the precipitation of Mg(OH)2. Consultation of the solubility guidelines in the experiment reveals that the Ca(OH)2 of slaked lime is moderately soluble in water. Hence, it can dissociate in water to give one Ca2+ ion and two OH- ions for each unit of Ca(OH)2 that dissolves. The OH- ions react with Mg2+ ions in the water to form the insoluble precipitate. The Ca2+ ions are unaffected by this reaction, and so we do not include them in the net ionic reaction (Equation 2). They are removed by the separate reaction with CO32- ions from the soda ash.
(2)
Household water softeners typically use a different process, known as ion exchange. Ion-exchange devices consist of a bed of plastic (polymer) beads covalently bound to anion groups, such as -COO-. The negative charge of these anions is balanced by Na+ cations attached to them. When water containing Ca2+ and Mg2+ is passed through the ion exchanger, the Ca2+ and Mg2+ ions are more attracted to the anion groups than the Na+ ions. Hence, they replace the Na+ ions on the beads, and so the Na+ ions (which do not form scale) go into the water in their place.
Figure 1
When hard tapwater passes through the ion exchanger (left), the calcium ions from the tapwater replace the sodium ions in the ion exchanger. The softened water, containing sodium ions in place of calcium ions, can be collected for household use.
Unfortunately, many people with high blood pressure or other health problems must restrict their intake of sodium. Because water softened by this type of ion exchange contains many sodium ions, people with limited sodium intakes should avoid drinking water that has been softened this way. Several new techniques for softening water without introducing sodium ions are beginning to appear on the market.
 www.premierwatermn.com
www.premierwatermn.com